Abstract
Aims
We investigated anticholinergic medicines use among older adults initiating dementia medicines.
Methods
We used Pharmaceutical Benefits Scheme dispensing claims to identify patients who initiated donepezil, rivastigmine, galantamine or memantine between 1 January 2013 and 30 June 2017 (after a period of ≥180 days with no dispensing of these medicines) and remained on therapy for ≥180 days (n = 4393), and dispensed anticholinergic medicines in the 180 days before and after initiating dementia medicines. We further examined anticholinergic medicines prescribed by a prescriber other than the one initiating dementia medicines.
Results
One‐third of the study cohort (1439/4393) was exposed to anticholinergic medicines up to 180 days before or after initiating dementia medicines. Among patients exposed to anticholinergic medicines, 46% (659/1439) had the same medicine dispensed before and after initiating dementia medicines. The proportion of patients dispensed anticholinergic medicines increased by 2.5% (95% confidence interval [CI]: 1.3–3.7) after initiating dementia medicines. Antipsychotics use increased by 10.1% (95% CI: 7.6–12.7) after initiating dementia medicines; driven by increased risperidone use (7.3%, 95% CI: 5.3–9.3). Nearly half of patients dispensed anticholinergic medicines in the 180 days after (537/1133), were prescribed anticholinergic medicines by a prescriber other than the one initiating dementia medicines.
Conclusion
Use of anticholinergic medicines is common among patients initiating dementia medicines and this occurs against a backdrop of widespread campaigns to reduce irrational medicine combinations in this vulnerable population. Decisions about deprescribing medicines with questionable benefit among patients with dementia may be complicated by conflicting recommendations in prescribing guidelines.
Keywords: anticholinergics, Australia, cholinesterase inhibitors, dementia, older adults, pharmacoepidemiology, potentially inappropriate medicines
What is already known about this subject
Adverse effects of anticholinergic medicines are exaggerated in older adults with dementia and may worsen symptoms of dementia.
Recent campaigns have encouraged clinicians to put more focus on appropriate use of medicines for older adults.
What this study adds
Despite their antagonistic pharmacological effects, current real‐world evidence shows that 1/3 of patients initiating dementia medicines were exposed to anticholinergic medicines up to 180 days before or after.
There is a myriad of guidelines on managing symptoms of dementia; however, deprescribing medicines with questionable benefit among patients with dementia are complicated by conflicting recommendations, especially approaches to manage co‐existing morbidities.
1. INTRODUCTION
Nearly 50 million people worldwide are currently living with dementia.1, 2 Cholinesterase inhibitors and memantine (dementia medicines) are the approved pharmacological mainstays and part of the standard care for managing the symptoms of dementia but they do not reverse or stop its progression.3, 4
Dementia medicines have the potential to cause agitation, insomnia and incontinence5 and these unwanted effects might lead to initiation or increased use of anticholinergic medicines.6, 7, 8, 9 Additionally, older adults with dementia are commonly prescribed medicines with anticholinergic properties to treat comorbidities such as depression, and anxiety and this further complicates therapy with dementia medicines.10 Anticholinergic medicines may cause cognitive impairment, confusion, loss of concentration and delirium, which are exaggerated in older adults with dementia.5, 11, 12, 13 Moreover, use of anticholinergic medicines may reduce the therapeutic effects of dementia medicines. A recent population‐based study investigating the effect of high anticholinergic burden after starting cholinesterase inhibitors found that concomitantly use of anticholinergic medicines had a negative impacted on the treatment response to cholinesterase inhibitors, leading to treatment modification, delirium and mortality.14
In an attempt to reduce variation in the management of dementia and to improve quality of care, best practice guidelines including those of the National Institute for Health and Care Excellence and American Psychiatric Association now recommend medicine reviews at the time of diagnosis to reduced irrational medicine use in patients with dementia.15, 16, 17, 18, 19 These reviews also provide an opportunity to identify and rule out any reversible cause of confusion such as adverse effects of other medicines or untreated comorbidities.20 Recommendations on improving clinical outcomes have been updated; however, decisions on reducing potentially inappropriate combinations of medicines, such as concomitant use of dementia and anticholinergic medicines are challenging due to lack of clarity in guidelines for managing comorbidities in patients with dementia.16
In Australia, few studies have investigated the use of anticholinergic medicines at the time of initiating dementia therapy.21, 22 Most recently, Gadzhanova and colleagues22 used 2009–2010 dispensing data to show that approximately 1/3 of patients initiating dementia medicines are exposed to anticholinergic medicines, in particular, antipsychotics.21, 22 Since then, guideline recommendations have been updated to increase awareness of concomitant medicines use and its impact on the clinical outcomes in patients with dementia and a number of campaigns such as Choosing Wisely Australia® have encouraged clinicians to put more focus on appropriate use of medicines for older Australians.23 This calls for updated evidence of anticholinergic medicines exposure among patients with dementia to further guide policy on prescribing practices.
In this study we aimed to provide contemporary real‐world evidence of anticholinergic medicines use among patients initiating dementia therapy. We examined which anticholinergic medicines were most commonly dispensed before and after initiating dementia medicines. We further sought to identify if patients were prescribed anticholinergic medicines by a prescriber other than the one initiating pharmacological treatment for dementia.
2. METHODS
2.1. Data source and study setting
In Australia, all citizens and permanent residents are entitled to subsidised access to prescribed medicines through the Pharmaceutical Benefits Scheme (PBS). We used anonymised nationwide 10% random sample of PBS‐eligible people and their dispensing history. This standard dataset is provided by the Australian Government Department of Human Services for analytical use and has been previously described in detail.24 It captures information on all dispensed PBS‐listed medicines including patient age, sex, date of dispensing, prescriber information and other variables. All medicines are uniquely identified by item codes and the Anatomical Therapeutic Chemical (ATC) classification codes. PBS item code provides medicine details at the product level, including generic name, form, strength, administration route, quantity per unit (pack size) and approved indication, where applicable.24
We extracted data from 1 July 2012 through to 31 December 2017 and examined cholinesterase inhibitors or memantine dispensings from 1 January 2013 to 30 June 2017 (study period).
2.2. Medicines of interest
We included 3 cholinesterase inhibitors subsidised under PBS for the management of cognitive impairment in patients with mild to moderately severe Alzheimer disease: donepezil (ATC code N06DA02), rivastigmine (ATC code N06DA03) and galantamine (ATC code N06DA04). We also included memantine (ATC code N06DX01), a N‐methyl‐d‐aspartate receptor inhibitor which is subsidised for patients with moderately severe to severe Alzheimer disease.
There is a wide range of medicines with anticholinergic effects. We limited the selection of these medicines based on a previously published list by Kalisch Ellett and colleagues13 who used medicines listed on the anticholinergic risk scale and the anticholinergic drug scale to show associations between anticholinergic medicine use and risk of hospitalisation with confusion or dementia.13 Table S1 lists these medicines, their ATC codes, therapeutic use and medicine class.
2.3. Study cohort and medicines exposure
Our study comprised all adults in the PBS 10% sample, aged ≥60 years, who were initiated cholinesterase inhibitors or memantine during the study period and continued dispensings for at least 180 days. We defined initiation of therapy to be the first dementia medicines dispensing after a period of at least 180 days, during which no dispensing of any dementia medicines had occurred.
We assumed that the person had remained on therapy for at least 180 days, if they had ≥5 dispensings of their dementia medicines within 180 days after the date of initiation. We required ≥5 dispensings for donepezil, galantamine and memantine 20‐mg tablets as they are available in 28‐day pack sizes for once daily dosing and oral formulations of rivastigmine are available in 56‐capsule packs for twice daily dosing. For memantine 10‐mg tablets, we required only ≥3 dispensings, as the available pack size is 56‐tablet packs and can be used as once‐daily dosing.25
We defined exposure to anticholinergic medicines before dementia therapy as any dispensing of an anticholinergic medicine 1–180 days prior to initiating dementia medicines. We defined exposure to anticholinergic medicines after initiation of dementia therapy as any dispensing of an anticholinergic within 180 days after initiating dementia medicines (i.e. including same day dispensings of an anticholinergic medicine with cholinesterase inhibitors or memantine). Figure S1 demonstrates the selection of the study cohort and their exposure to medicines with anticholinergic effects from the period 180 days before and until 180 days after.
2.4. Data analysis
We described the patient characteristics, their exposure to anticholinergic medicines and calculated the number and proportion of patients exposed to anticholinergic medicines before and after initiating dementia medicines by medicine class (antidepressants, anti‐Parkinson's, antipsychotics and other anticholinergic) and specific medicines. We also estimated the number of patients with anticholinergic medicine dispensings by each 30‐day period up to 180 days before and 180 days after initiating dementia medicines. We used the McNemar's test to estimate the difference between the proportion of patients dispensed anticholinergic medicines from before to after initiation of dementia medicines. Finally, we examined the proportion of patients prescribed at least 1 anticholinergic medicine written by a prescriber other than the one who initiated the dementia medicines.
We used SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata Release 14 (College Station, TX: StataCorp LP) to conduct all data analysis.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.26
3. RESULTS
Our cohort comprised 4393 older adults who initiated dementia medicines and remained on therapy for at least 180 days. Just over half (56%) of the cohort were female, 70% were aged between 75 and 89 years and 69% initiated donepezil as their dementia treatment (Table 1).
Table 1.
Characteristics of the cohort of patients initiating dementia medicines by exposure to anticholinergic medicines
| Study cohort | Exposed to anticholinergic medicines | Unexposed to anticholinergic medicines | ||||
|---|---|---|---|---|---|---|
| Characteristics | n = 4393 | % | n = 1439 | % | n = 2954 | % |
| Sex | ||||||
| Female | 2467 | 56.2 | 853 | 59.3 | 1614 | 54.6 |
| Male | 1926 | 43.8 | 586 | 40.7 | 1340 | 45.4 |
| Age categories (y) | ||||||
| 60–64 | 157 | 3.6 | 35 | 2.4 | 122 | 4.1 |
| 65–69 | 288 | 6.6 | 103 | 7.2 | 185 | 6.3 |
| 70–74 | 582 | 13.3 | 158 | 11.0 | 424 | 14.4 |
| 75–79 | 997 | 22.7 | 329 | 22.9 | 668 | 22.6 |
| 80–84 | 1136 | 25.6 | 379 | 26.3 | 757 | 25.6 |
| 85–89 | 925 | 21.1 | 317 | 22.0 | 608 | 20.6 |
| 90–94 | 274 | 6.2 | 104 | 7.2 | 170 | 5.8 |
| 95–99 | 34 | 0.8 | 14 | 1.0 | 20 | 0.7 |
| Dementia medicines initiated | ||||||
| Donepezil | 3023 | 68.8 | 925 | 64.3 | 2098 | 71.0 |
| Rivastigmine | 557 | 12.7 | 229 | 15.9 | 328 | 11.1 |
| Galantamine | 491 | 11.2 | 140 | 9.7 | 351 | 11.9 |
| Memantine | 322 | 7.3 | 145 | 10.1 | 177 | 6.0 |
One‐third of the study cohort (1439/4393) was exposed to least 1 anticholinergic medicine in the 180 days before or after initiating dementia medicines. Of those exposed, nearly 2/3 (59%) were dispensed antipsychotic medicines. Risperidone was the most common anticholinergic medicine dispensed (29% of those exposed), followed by prochlorperazine (18%), amitriptyline (16%), quetiapine (14%) and oxybutynin (13%).
The proportion of patients exposed to anticholinergic medicines in each 30‐day period ranged from 9.7 to 10.7% during the 180 days before initiating dementia medicines and from 13.0 to 13.5% after initiating dementia medicines (Figure 1).
Figure 1.
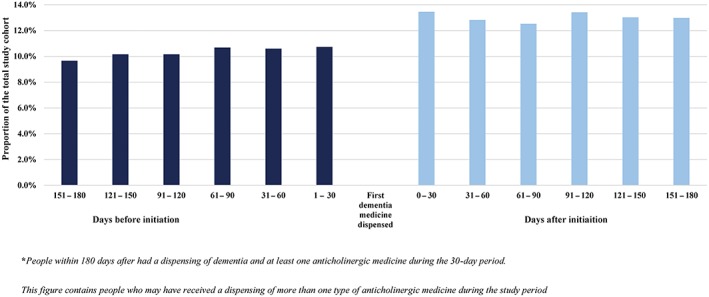
Proportion of patients dispensed anticholinergic medicines by each 30‐day interval from 180 days before to 180 days after initiating dementia medicines
We observed a 2.5% absolute increase in the number of patients dispensed anticholinergic medicines after initiating dementia medicines with the largest increase in the concomitant dispensing of antipsychotics (10.1%). We found that more patients were dispensed risperidone and quetiapine after initiating dementia medicines (7.3 and 2.3% respectively) than during the 180 days before such therapy (Table 2).
Table 2.
Number and difference in proportions of older adults exposed to anticholinergic medicines 180 days before or 180 days after initiating dementia medicines by medicine class and the most commonly dispensed anticholinergic medicines
| Anticholinergic medicines exposure: | Before initiating dementia medicines | After initiating dementia medicines | Difference in proportions | |||
|---|---|---|---|---|---|---|
| n = 1439 | n = 1023 | % | n = 1133 | % | % | CI |
| Anticholinergic medicine class a | ||||||
| Antidepressants | 335 | 23.3 | 316 | 22.0 | −1.3 | −2.9 to 0.3 |
| Anti‐Parkinson's | 25 | 1.7 | 26 | 1.8 | 0.1 | −0.5 to 0.6 |
| Antipsychotics | 600 | 41.7 | 746 | 51.8 | 10.1 | 7.6 to 12.7 |
| Other anticholinergic medicines | 453 | 31.5 | 459 | 31.9 | 0.4 | −2.1 to 2.9 |
| Most common anticholinergic medicines | ||||||
| Risperidone | 257 | 17.9 | 362 | 25.2 | 7.3 | 5.3 to 9.3 |
| Prochlorperazine | 176 | 12.2 | 161 | 11.2 | −1.0 | −2.9 to 0.8 |
| Amitriptyline | 186 | 12.9 | 171 | 11.9 | −1.0 | −2.4 to 0.3 |
| Quetiapine | 161 | 11.2 | 194 | 13.5 | 2.3 | 1.2 to 3.4 |
| Oxybutynin | 144 | 10.0 | 143 | 9.9 | −0.07 | −1.3 to 1.2 |
| Olanzapine | 115 | 8.0 | 124 | 8.6 | 0.6 | −0.3 to 1.5 |
| Loperamide | 74 | 5.1 | 89 | 6.2 | 1.0 | −0.02 to 2.1 |
| Paroxetine | 72 | 5.0 | 78 | 5.4 | 0.4 | −0.1 to 1.0 |
| Haloperidol | 44 | 3.1 | 47 | 3.3 | 0.2 | −0.7 to 1.1 |
| Carbamazepine | 15 | 1.0 | 38 | 2.6 | 0.3 | −0.2 to 0.8 |
CI, 95% confidence interval.
(%) presented are proportions of all patients in the cohort dispensed an anticholinergic medicine (n = 1439).
This table contains patients who may have received a dispensing of >1 type of anticholinergic medicines during the study period.
Table S1 outlines the anticholinergic medicine classes.
Among patients exposed to an anticholinergic medicine, approximately 1/3 (416/1439) commenced treatment after initiating dementia medicines, while nearly half (659/1439) had the same anticholinergic medicine dispensed before and after initiating dementia medicines. Risperidone was most commonly dispensed (37.5%) among those who commenced anticholinergic medicines after initiation of dementia therapy (Table 3), and among those with exposure to same anticholinergic medicines (22.8%) before and after. (Table S2).
Table 3.
Most common anticholinergic medicines commenced by older adults within 180 days after initiating dementia medicines
| Anticholinergic medicines | Patients with new exposure to anticholinergic medicines | |
|---|---|---|
| Any anticholinergic medicine | n = 416 | % |
| Risperidone | 156 | 37.5 |
| Prochlorperazine | 76 | 18.3 |
| Quetiapine | 47 | 11.3 |
| Amitriptyline | 38 | 9.1 |
| Oxybutynin | 36 | 8.7 |
| Loperamide | 34 | 8.2 |
| Olanzapine | 23 | 5.5 |
| Haloperidol | 19 | 4.6 |
| Paroxetine | 9 | 2.2 |
| Carbamazepine | 7 | 1.7 |
(%) presented are proportions of all patients dispensed anticholinergic medicines for the first time after initiating dementia medicines (n = 416).
This table contains patients who may have received a dispensing of >1 type of anticholinergic medicine during the study period.
Among patients who were dispensed anticholinergic medicines after initiating dementia medicines (with or without prior exposure), we found that nearly half (537/1133) had their anticholinergic medicines prescribed by a prescriber other than the one who initiated dementia therapy.
4. DISCUSSION
Dementia is a progressive disease and medicines for dementia might slow the cognitive decline. Our study provides insight into recent real‐world use of medicines for dementia and the prescribing of medicines with anticholinergic effects in this population. Current recommendations encourage a medicine review at the time of dementia diagnosis and thereafter to reduce potentially inappropriate medicine use, yet we found that anticholinergic medicines were commonly dispensed to our cohort.
The results of our study mirror the findings of previous studies conducted almost a decade ago using PBS dispensing data,21, 22 suggesting that there has been little change in prescribing practices among patients dispensed dementia medicines. These results occur against a backdrop of increasing emphasis on the importance of evidence‐based practice and calls for improved prescribing in this vulnerable population. Our findings are also comparable to international studies. In a very recent systematic review, Hukins and colleagues identified anticholinergic medicines as among the most commonly prescribed potentially inappropriate medicines among patients with dementia, with rates of prescriptions ranging from 6 to 46%.27
There is a myriad of guidelines on managing symptoms of dementia; however, they often lack depth, consistency and/or consensus and the overall recommendations on managing comorbidities are generally weak and/or based upon insufficient evidence.28 This leads to increased challenges for clinicians with ever‐extensive clinical responsibilities and makes it difficult to determine what approach to take. For instance, concurrent use of anticholinergic medicines is not recommended with cholinesterase inhibitors; however, the primary pharmacological management for behavioural and psychological disturbances in dementia are antipsychotics such as risperidone.15, 18, 28, 29, 30, 31, 32
In our study, the antipsychotic risperidone was the most commonly dispensed medicine with anticholinergic effects both before and after initiating dementia medicines. Under PBS restrictions, risperidone formulations are only eligible for government subsidy for managing behavioural and psychological symptoms of dementia; thus accounting for the substantial number of dispensings captured in our data.33 The safety and efficacy of risperidone and other commonly dispensed antipsychotics such as quetiapine and olanzapine use in patients with dementia remains uncertain.30, 34, 35, 36 Clinical trials such as the CATIE‐AD study conclude that adverse effects offset advantages in the efficacy of antipsychotics in the management of psychosis, aggression or agitation in patients with Alzheimer disease.37
Other anticholinergic medicines commonly co‐dispensed among our study cohort, such as prochlorperazine, loperamide, oxybutynin and amitriptyline, may have been used to manage the adverse effects of dementia medicines, such as nausea, diarrhoea, agitation, insomnia and incontinence.5 This suggests that the initiation of dementia medicines may have led to a prescribing cascade7 and use of medicines with the potential to worse cognition, among other adverse effects. This might be most obvious with the prescription of the anticholinergic medicine oxybutynin to manage urinary symptoms that results from therapy with dementia medicines.11
Patients with dementia experience more comorbidities, may receive a higher number of medicines and visit multiple prescribers in comparison to their cognitively intact counterparts, which leads to fragmentation of care.27, 38, 39 In our study, we found that nearly half of the cohort who were co‐prescribed anticholinergic and dementia medicines visited multiple prescribers. This may have contributed to polypharmacy and irrational combinations of pro‐cholinergic and anticholinergic medicines. For example, drug‐interaction software can only work if both medicines are recorded in the same system, and prescribers would be familiar with the medicines they choose to prescribe but might be ignorant of others.
Our study has some notable limitations. Medicines with anticholinergic effects have multiple indications and, from our dataset, we could not determine the reason for which they were prescribed. Thus, we were unable to confirm for each case, if anticholinergic medicines use could have been avoided. In addition, we did not have dosing information and hence could not measure dose reductions of specific anticholinergic medicines after a patient initiated dementia medicines.
5. CONCLUSION
Anticholinergic medicine use among patients living with dementia needs further attention from clinicians. Despite high‐quality evidence of the risks associated with their use, anticholinergic medicines continue to be commonly dispensed in this vulnerable population. Decisions about the use of medicines with questionable benefit among patients with dementia are complicated by conflicting recommendations in prescribing guidelines, especially approaches to manage co‐existing morbidities. Periodic monitoring such as medicine reviews at the time of dementia diagnosis and thereafter may help prescribers identify and reduce potentially inappropriate medicine use and its detrimental effects.
CONFLICT OF INTEREST
A.J.M. receives funding from GlaxoSmithKline for a postgraduate scholarship for a student under his supervision. S.A.P. is a member of the Drug Utilisation Sub‐Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the committee.
CONTRIBUTORS
S.W.N., S.A.P., D.G.L., N.B., A.J.M., and H.Z. conceived and designed the study. S.W.N. and M.L. conducted the statistical analysis. SN drafted the initial manuscript; all authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors commented on drafts, approved the final manuscript, and take responsibility for the integrity of the data and accuracy. of the data analysis.
6.
Supporting information
Figure S1. Selection of the study cohort and their exposure to medicines with anticholinergic effects from the period 180 days before and until 180 days after initiating dementia medicines.
Table S1: Anticholinergic medicines included in this study listed by their World Health Organisation (WHO) Anatomical Therapeutic Chemical (ATC) classification codes and medicine class
Table S2: Same anticholinergic medicines dispensed to older adults during both time periods: within 180 days before and within 180 days after initiating dementia medicines (n = 659)
ACKNOWLEDGEMENTS
We thank the Australian Government Department of Human Services for providing the data. Jan Zirk‐Sadowski contributed to the early drafts of the statistical plan and preliminary data analysis.
This research is funded by the Australian National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Medicines and Ageing (ID: 1060407), a Cooperative Research Centre Project (CRC‐P) Grant from the Australian Government Department of Industry, Innovation and Science (ID: CRC‐P‐439) and philanthropic support from Mr Ross Brown AM. Dr Zoega is supported by a Scientia Fellowship from the University of New South Wales. The views expressed in this study are those of the authors only.
Narayan SW, Pearson S‐A, Litchfield M, et al. Anticholinergic medicines use among older adults before and after initiating dementia medicines. Br J Clin Pharmacol. 2019;85:1957–1963. 10.1111/bcp.13976
The authors confirm that the PI for this paper is Dr Sujita W. Narayan. This is not a clinical study.
Data Availability Statement:Approval for this study was obtained from the New South Wales Population and Health Services Research Ethics Committee (protocol number: PHSREC #: 2013/11/494). Analyses of the PBS 10% sample was approved by the Australian Government Department of Human Services External Request Evaluation Committee (reference MI8554). Restrictions apply to the availability of these data, which were used under approval for this study.
DATA AVAILABILITY STATEMENT
Approval for this study was obtained from the New South Wales Population and Health Services Research Ethics Committee (protocol number: PHSREC #: 2013/11/494). Analyses of the PBS 10% sample was approved by the Australian Government Department of Human Services External Request Evaluation Committee (reference MI8554). Restrictions apply to the availability of these data, which were used under approval for this study.
REFERENCES
- 1. Dear BF, Gandy M, Karin E, et al. The pain course: 12‐ and 24‐month outcomes from a randomized controlled trial of an internet‐delivered pain management program provided with different levels of clinician support. J Pain. 2018;19(12):1491‐1503. [DOI] [PubMed] [Google Scholar]
- 2. Walco GA, Kopecky EA, Weisman SJ, et al. Clinical trial designs and models for analgesic medications for acute pain in neonates, infants, toddlers, children, and adolescents: ACTTION recommendations. Pain. 2018;159(2):193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anticholinesterases in Alzheimer's disease . Australian Medicines Handbook, 2018. Adelaide: Australian Medicines Handbook Pty Ltd; 2018. [Google Scholar]
- 4. Mayeux R, Sano M. Treatment of Alzheimer's disease. N Engl J Med. 1999;341(22):1670‐1679. [DOI] [PubMed] [Google Scholar]
- 5. Mohammad D, Chan P, Bradley J, Lanctot K, Herrmann N. Acetylcholinesterase inhibitors for treating dementia symptoms ‐ a safety evaluation. Expert Opin Drug Saf. 2017;16(9):1009‐1019. [DOI] [PubMed] [Google Scholar]
- 6. Roe CM, Anderson MJ, Spivack B. Use of anticholinergic medications by older adults with dementia. J am Geriatr Soc. 2002;50(5):836‐842. [DOI] [PubMed] [Google Scholar]
- 7. Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808‐813. [DOI] [PubMed] [Google Scholar]
- 8. Modi A, Weiner M, Craig BA, Sands LP, Rosenman MB, Thomas J 3rd. Concomitant use of anticholinergics with acetylcholinesterase inhibitors in Medicaid recipients with dementia and residing in nursing homes. J am Geriatr Soc. 2009;57(7):1238‐1244. [DOI] [PubMed] [Google Scholar]
- 9. Sverdrup Efjestad A, Ihle‐Hansen H, Hjellvik V, Blix HS. Comedication and treatment length in users of acetylcholinesterase inhibitors. Dement Geriatr Cogn Dis Extra. 2017;7(1):30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gnjidic D, Agogo GO, Ramsey CM, Moga DC, Allore H. The impact of dementia diagnosis on patterns of potentially inappropriate medication use among older adults. J Gerontol a Biol Sci Med Sci. 2018;73(10):1410‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Triantafylidis LK, Clemons JS, Peron EP, Roefaro J, Zimmerman KM. Brain over bladder: a systematic review of dual cholinesterase inhibitor and urinary anticholinergic use. Drugs Aging. 2018;35(1):27‐41. [DOI] [PubMed] [Google Scholar]
- 12. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalisch Ellett LM, Pratt NL, Ramsay EN, Barratt JD, Roughead EE. Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J am Geriatr Soc. 2014;62(10):1916‐1922. [DOI] [PubMed] [Google Scholar]
- 14. Ah YM, Suh Y, Jun K, Hwang S, Lee JY. Effect of anticholinergic burden on treatment modification, delirium and mortality in newly diagnosed dementia patients starting a cholinesterase inhibitor: a population‐based study. Basic Clin Pharmacol Toxicol. 2019;124(6):741‐748. 10.1111/bcpt.13184 [DOI] [PubMed] [Google Scholar]
- 15. Freo U, Furnari M, Ori C. Effects of tapentadol on pain, motor symptoms and cognitive functions in Parkinson's disease. J Pain Res. 2018;11:1849‐1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbett A, Burns A, Ballard C. Don't use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ. 2014;349:g6420. [DOI] [PubMed] [Google Scholar]
- 17. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950‐960. [DOI] [PubMed] [Google Scholar]
- 18. Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pink J, O'Brien J, Robinson L, Longson D, Guideline Committee . Dementia: assessment, management and support: summary of updated NICE guidance. BMJ. 2018;361:k2438. [DOI] [PubMed] [Google Scholar]
- 20. Woudstra FH, van de Poel‐Mustafayeva AT, van der Ploeg MV, de Vries JJ, van der Lek RFR, Izaks GJ. Symptoms mimicking dementia in a 60‐year‐old woman with bipolar disorder: a case report. BMC Res Notes. 2014;7(1):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robinson M, Rowett D, Leverton A, Mabbott V. Changes in utilisation of anticholinergic drugs after initiation of cholinesterase inhibitors. Pharmacoepidemiol Drug Saf. 2009;18(8):659‐664. [DOI] [PubMed] [Google Scholar]
- 22. Gadzhanova S, Roughead E, Robinson M. Use of medicines with anticholinergic and sedative effect before and after initiation of anti‐dementia medications. Drugs ‐ Real World Outcomes. 2015;2(1):53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webster L, Diva U, Tummala R, Sostek M. Treatment with Naloxegol versus placebo: pain assessment in patients with noncancer pain and opioid‐induced constipation. Pain Pract. 2018;18(4):505‐514. [DOI] [PubMed] [Google Scholar]
- 24. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian pharmaceutical benefits scheme data collection: a practical guide for researchers. BMC Res Notes. 2015;8(1):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antonow JA, Smith AB, Silver MP. Medication error reporting: a survey of nursing staff. J Nurs Care Qual. 2000;15(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 26. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hukins D, Macleod U, Boland JW. Identifying potentially inappropriate prescribing in older people with dementia: a systematic review. Eur J Clin Pharmacol. 2019;75(4):467‐481. [DOI] [PubMed] [Google Scholar]
- 28. Ngo J, Holroyd‐Leduc JM. Systematic review of recent dementia practice guidelines. Age Ageing. 2015;44(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 29. Pergolizzi JV, LeQuang JA, Berger GK, Raffa RB. The basic pharmacology of opioids informs the opioid discourse about misuse and abuse: a review. Pain Ther. 2017;6(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frank L, Kleinman L, Ciesla G, Rupnow MF, Brodaty H. The effect of risperidone on nursing burden associated with caring for patients with dementia. J am Geriatr Soc. 2004;52(9):1449‐1455. [DOI] [PubMed] [Google Scholar]
- 31. Kongpakwattana K, Sawangjit R, Tawankanjanachot I, Bell JS, Hilmer SN, Chaiyakunapruk N. Pharmacological treatments for alleviating agitation in dementia: a systematic review and network meta‐analysis. Br J Clin Pharmacol. 2018;84(7):1445‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee PE, Gill SS, Freedman M, Bronskill SE, Hillmer MP, Rochon PA. Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. BMJ. 2004;329(7457):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng L, Cashman J. The management of acute pain. Medicine (UK). 2018;46(12):780‐785. [Google Scholar]
- 34. Rossom RC, Rector TS, Lederle FA, Dysken MW. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia. J am Geriatr Soc. 2010;58(6):1027‐1034. [DOI] [PubMed] [Google Scholar]
- 35. Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off‐label uses in adults: a systematic review and meta‐analysis. JAMA. 2011;306(12):1359‐1369. [DOI] [PubMed] [Google Scholar]
- 36. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. 2012;169(9):900‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355(15):1525‐1538. [DOI] [PubMed] [Google Scholar]
- 38. Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eggers T, Norberg A, Ekman SL. Counteracting fragmentation in the care of people with moderate and severe dementia. Clin Nurs Res. 2005;14(4):343‐369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Selection of the study cohort and their exposure to medicines with anticholinergic effects from the period 180 days before and until 180 days after initiating dementia medicines.
Table S1: Anticholinergic medicines included in this study listed by their World Health Organisation (WHO) Anatomical Therapeutic Chemical (ATC) classification codes and medicine class
Table S2: Same anticholinergic medicines dispensed to older adults during both time periods: within 180 days before and within 180 days after initiating dementia medicines (n = 659)
Data Availability Statement
Approval for this study was obtained from the New South Wales Population and Health Services Research Ethics Committee (protocol number: PHSREC #: 2013/11/494). Analyses of the PBS 10% sample was approved by the Australian Government Department of Human Services External Request Evaluation Committee (reference MI8554). Restrictions apply to the availability of these data, which were used under approval for this study.


