Abstract
Tyrosine kinase receptors are transmembrane proteins involved in cell signaling and interaction. Among them, the TAM family (composed by Tyro 3, Axl, and Mer) represents a peculiar subgroup with an important role in many physiological and pathological conditions. Despite different mechanisms of activation (e.g., protein S and Galactin-3), TAM action is tightly related to their common ligand, a protein named growth arrest-specific 6 (Gas6). Since the expression of both TAM and Gas6 is widely distributed among tissues, any alteration of one of these components can lead to different pathological conditions. Moreover, as they are indispensable for homeostasis maintenance, in recent years a growing interest has emerged regarding their role in the regulation of the inflammatory process. Due to this involvement, many authors have demonstrated the pivotal role of the Gas6/TAM axis in both sepsis and the sepsis-related inflammatory responses. In this narrative review, we highlight the current knowledge as well as the last discoveries on TAM and Gas6 implication in different clinical conditions, notably in sepsis and septic shock. Lastly, we underline not only the feasible use of Gas6 as a diagnostic and prognostic biomarker in certain systemic acute conditions but also its potential therapeutic role in these life-threatening diseases.
1. Brief “TAM” Story
Tyrosine kinase receptors (RTKs) are transmembrane proteins often implicated in cell-to-cell communication. Until now, 58 RTKs have been identified [1]; these receptors pilot, through phosphorylation, an enormous amount of essential signaling pathways, regulating proliferation, survival, and apoptosis.
Among RTKs, Tyro3, Axl, and Mer (gene name Mertk) share structural similarity (notably two Ig-like domains, two fibronectin type III domains, a hydrophobic transmembrane domain, and a tyrosine kinase domain) and they are grouped in the so-called “TAM family” (Figure 1). Despite their deep resemblance, TAM receptors are expressed by different cell types and tissues (Table 1): Tyro3 is generally localized in the nervous system, whereas Mer and Axl have been found in different tissues and they are frequently coexpressed by the same cells [2]. This coexpression can be either equivalent in some cells, such as Kupffer cells in the liver and red pulp macrophages in the spleen, or unbalanced in others, such as for CD68+ tingible macrophages, which are primarily Mer+, and CD11c+ white pulp dendritic cells (DCs), which are mostly Axl+ [3].
Figure 1.
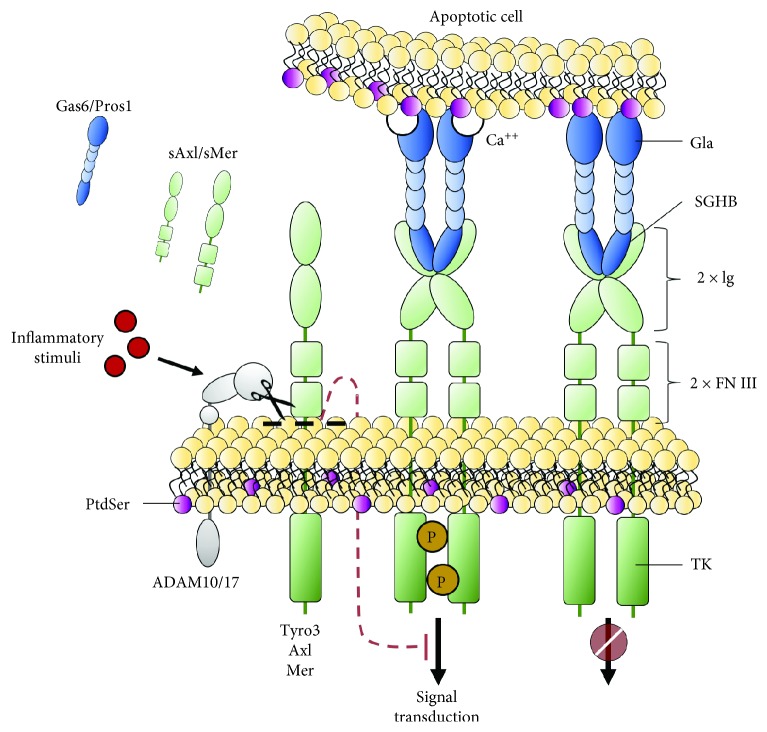
TAM structures and posttranslational regulation. Schematic representation of TAM receptors and their ligands. All TAM receptors share structural domains, i.e., the tyrosine kinase (TK) domain, the transmembrane domain, two fibronectin type III domains (FN III), and two Ig-like domains (Ig) from the C-terminal to the N-terminal (right). The TAM ligands Gas6 and Pros1 share a sex hormone-binding globulin (SHBG) domain and a gamma-carboxyglutamic acid-rich (Gla) domain (right). The Gla domain binds phosphatidylserine (PtdSer) exposed in the outer/external side of the apoptotic cell plasma-membrane, while the SHBG domain interacts with TAM receptor Ig-like domains on the surface of TAM-expressing cells, thus acting as “bridge” proteins (right). The binding itself does not result in receptor activation that occurs through receptor transphosphorylation and in a Ca++-dependent fashion (center). For Mer and Axl, the signal transduction is shut down by proteolytic cleavage of the receptor ectodomain (shedding), which is mediated by the transmembrane disintegrin and metalloproteinase (ADAM) 17 and/or ADAM10. Shedding can be induced by inflammatory stimuli (e.g., lipopolysaccharide) leading to the extracellular domain release of the receptor and generating a soluble Axl (sAxl) and soluble Mer (sMer) form able to interact with and sequester the ligands Gas6 and Pros1 (left).
Table 1.
The widespread expression of the TAM receptor.
| Tyro3 | Axl | Mer | |
|---|---|---|---|
| Brain | (i) Microglial cells [4] (ii) Astrocytes [4] |
(i) Microglial cells [4] (ii) Astrocytes [4] |
(i) Microglial cells [4] (ii) Astrocytes [4] |
|
| |||
| Heart | (i) Cardiomyocytes [5] | ||
|
| |||
| Breast | (i) Mammary epithelial cells [6] | ||
|
| |||
| Lung | (i) Macrophages CD11blowCD11chigh [7] | (i) Alveolar macrophages [8] | |
|
| |||
| Liver | (i) Kupffer cells [9] | (i) Kupffer cells [9] (ii) HSCs (q/a) [9] (iii) LSECs [9] (iv) Hepatocytes [9] |
(i) Kupffer cells [9] (ii) HSC (a) [9] (iii) LSEC [9] |
|
| |||
| Spleen | (i) DCs CD11chigh [10] | (i) Macrophages F4/80high, B220–, CD11c+ and MHCII+ red pulp [11] (ii) Macrophages F4/80+CD68+ (tingible body) [11] |
|
|
| |||
| Kidney | (i) Podocytes [12] | (i) Podocytes [12] | |
|
| |||
| Testis | (i) Sertoli cells [13] | (i) Sertoli cells [13] | (i) Sertoli cellslow [13] (ii) Leydig cells [13] |
|
| |||
| Peritoneum | (i) Macrophages [14] | (i) Macrophages [14] | |
|
| |||
| Blood/BM derived | (i) Platelets [15] (ii) Monocyteslow (iii) Monocyte-derived macrophageslow [16] (iv) NK cells [17] (v) DC CD11c+ [18] |
(i) Platelets [15] (ii) Monocyteshigh (iii) Monocyte-derived macrophageslow [16] (iv) NK cells [17] (v) DC CD11c+ [18] |
(i) Platelets [15] (ii) Monocyteslow (iii) Monocyte-derived macrophageshigh [16] (iv) NK cells [17] (v) DC CD11c+ [18] (vi) DCs CD11b+ and B220+ [19] (vii) NKT cells [20] |
Italic shows TAM expression located in human cells; all the others were found in murine cells. BM derived: bone marrow derived; HSCs: hepatic stellate cells; LSECs: liver sinusoidal endothelial cells; DCs: dendritic cells; NK: natural killer; NKT: natural killer T.
TAM were discovered and cloned by several groups in the 90s [2]. In the first years from their discovery, their role in the maintenance of homeostatic balance through the regulation of the phagocytosis of apoptotic bodies (efferocytosis) was demonstrated [21]. Gradually, their role in the innate inflammatory response and in the regulation of cell proliferation and apoptosis was elucidated, leading to growing interest. In fact, a deficiency in TAM expression is related to autoimmunity diseases [2] and, oppositely, their overexpression or aberrant activation (i.e., gain-of-function mutations) is associated with the development and progression of cancer [22].
In this context, the complex network of TAM functions has been clarified in recent years, as it seems more linked to the environmental context, or “milieu,” rather than to the expressing cell/tissue, such as neurodegenerative diseases [23], autoimmune diseases, and cancer [24]. TAM activation, which occurs through tyrosine cross-phosphorylation, is normally mediated by the binding with their ligands, growth arrest-specific 6 (Gas6) and protein S (Pros1). Gas6 and Pros1 share in the C-terminal portion the “sex hormone-binding globulin (SHBG) domain,” which binds the TAM Ig-like domains. The N-terminal portion includes the γ-carboxylate “gamma-carboxyglutamic acid-rich (Gla) domain,” responsible for binding the phospholipid phosphatidylserine (PtdSer) in a Ca++-dependent reaction (Figure 1).
Gas6 is able to bind and activate all TAM receptors, while Pros1 can only bind Mer and Tyro3, without interacting with Axl. In 2014, Lew et al. published a detailed paper showing that Gas6 is capable of binding and activating all TAM, but the most powerful effect was observed following Axl activation. Moreover, both murine and human recombinant Pros1 can bind and activate murine Tyro3 and Mer (but not Axl) in vitro. Lastly, they showed that the PtdSer-binding Gla domain present on Gas6, PtdSer itself, and Ca++ are all essential to achieve a full receptor activation, but none of them is involved in receptor binding [25]. Interestingly, Gas6/Pros1-TAM receptor binding is not able to determine the receptor activation per se [25]; so all the conditions described above need to be fulfilled in order to trigger the numerous signal transduction pathways, such as phosphoinositide 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAP kinase), nuclear factor-light-chain-enhancer of activated B cells (NF-κB), signal transducer and activator of transcription protein (STAT), phospholipase C (PLC), growth factor receptor-bound protein 2 (Grb2), Raf-1, extracellular-signal-regulated kinase (ERK), and others [26–28]. Rothlin et al. demonstrated that TAM signaling triggers the expression of the suppressor of cytokine signaling proteins, SOCS1 and SOCS3. In fact, in dendritic cells from mice knockout for all three TAM receptors (TAM triple knockout; TAM TKO), the induction of SOCS1 was substantially impaired [29, 30].
Until now, different mutations on TAM receptors have been linked to defined genetic diseases: primarily many MerTK mutations were associated with retinal degenerations [31]. In particular, TAM receptors differ from other RTKs since we know from mouse models that TAM genes can be ablated without any major effect on embryonic development [32]. As a consequence, TAM TKO mice are indistinguishable from their wild-type (WT) counterparts and this aspect appears peculiar because usually the absence of expression of other RTKs leads to severe embryonic development impairment, with intrauterine death [33]. Although during the first three life-weeks no macroscopic difference can be observed between TAM TKO and TAM WT mice, after this period TAM TKO mice develop several degenerative phenotypes. Male TAM TKO mice are infertile in adult life, a condition that is related to impaired sexual development and spermatogenesis. Indeed, Sertoli cells express all three TAM receptors as well as both ligands, Gas6 and Pros1, which allow them to manage, in an autocrine fashion, the phagocytosis of apoptotic germ cells (around 108/day in human male) [34]. The absence of TAM receptors results in incorrect efferocytosis and accumulation of apoptotic cells, damaging sexual organs. Still, both in adult TAM TKO and single Mer−/− mice, the impairment of phagocytosis causes the accumulation of apoptotic debris in the retina, causing a nearly complete absence of photoreceptors [35, 36] and blindness [32].
Since one of the main functions of TAM receptors is to modulate the immune homeostasis [2, 37], it is reasonable to consider their implication in autoimmune phenotypes. Qi et al. have demonstrated that TAM TKO mice develop a spontaneous liver disease which resembles autoimmune hepatitis. These mice exhibited chronic hepatitis, with progressive inflammatory cell infiltration and elevated cytokine levels in the liver [38]. Moreover, TAM TKO mice displayed splenomegaly, lymphadenopathy, and lymphocyte infiltration in nearly all tissues around 4-6 weeks after birth [37]. Also coagulation was impaired with both thrombosis and hemorrhages, especially in the brain, as well as skin lesions and hemophilic-like phenotypes with swollen joints [37].
Additionally, these mice generate high levels of circulating autoantibodies directed against dsDNA, collagens, and phospholipids, such as cardiolipin, PtdSer, phosphatidylethanolamine, and phosphatidylinositol [37].
Thus, we can summarize that TAM TKO mice have an autoimmune phenotype with features comparable to systemic lupus erythematosus (SLE), psoriasis, and rheumatoid arthritis [2, 37]. Antigen-presenting cells (APCs) from TAM TKO mice have a dysregulated activity in response to inflammatory stimuli, demonstrating a reduced tolerogenic behavior with the hyperproduction of type 1 interferons, interleukin (IL) 12, and overexpression of MHC class II and CD86 [29, 37]. This expression pattern is consistent with the splenomegaly and lymphadenopathy observed in adult TAM TKO mice.
Despite their structural homology, following activation TAM receptor signaling is shut down in different ways: the signal desensitization that occurs through the shedding of the ectodomain by proteolytic cleavage was reported for Mer and Axl [39, 40]. In spite of soluble Tyro3 increasing levels in the bloodstream in different chronic diseases [41, 42], this signal desensitization mechanism has not been described for Tyro3 yet (Figure 1). Between the TAM-common-fibronectin type III domains and the transmembrane domain, the proline residue Pro485 present in the Mer sequence makes it susceptible to cleavage by the metalloproteinase ADAM17, a disintegrin and metalloproteinase domain 17 [39], also known as tumor necrosis factor-alpha converting enzyme (TACE). Although the examination of the cleavage site sequence of several substrates shed by ADAM17 indicates that the distance between ADAM17 and its target is more important than the specific sequence in ectodomain shedding, the site direct mutagenesis of the Pro485 cleavage site results in Mer resistance to proteolysis [39].
The activation of pattern-recognition receptors (PRRs) with lipopolysaccharide (LPS) or polycytidylic acid (Poly:C) in macrophages results in the induction of cleavage of the Mer extracellular domain. Furthermore, LPS- and polyinosinic:polycytidylic acid- (PolyI:C-) induced Mer shedding is dependent on ADAM17, as it is abrogated in ADAM17 gene knockdown macrophages. Sather et al. have shown that the shedding of the Mer ectodomain results in the inactivation of the receptor and in additional neutralization of TAM ligands, which are sequestered by the released soluble form of the receptor ectodomain [43]. This autoregulatory mechanism is not exclusive to Mer but it has been described also for Axl. The cleavage, which generates the soluble and circulating Axl (sAxl), is induced by ADAM17 and another metalloproteinase, ADAM10 [44] (Figure 1). In 2010, Ekman et al. demonstrated that Gas6 is trapped by sAxl. In their elegant study, they hypothesized the absence of free-Gas6 circulating in the bloodstream in healthy subjects, since the molar concentration of sAxl is higher than the one of Gas6, thus suggesting that Gas6 released from cells is quickly bound by sAxl [45]. This seems related to the higher affinity of Gas6 for Axl in comparison to Mer. Indeed, Gas6 binds Axl with a dissociation constant in the subnanomolar range, whereas its affinity for Mer is at least 10-fold lower [46]. So, according to the interpretation suggested by Ekman et al., in the presence of Axl the interaction between Gas6 and Mer or soluble Mer (sMer) might be prevented. Conversely, a previous study published by Sather et al. demonstrated that both sAxl and sMer are able to inhibit the Gas6 activity. The authors focused on sMer, showing that the inactive sMer/Gas6-complex leads to a defective macrophage-mediated engulfment of apoptotic cells. Furthermore, they showed that the release of sMer is associated with a decrease of platelet aggregation in vitro and it could prevent the fatal collagen/epinephrine-induced thromboembolism in mice [43].
2. TAM Ligands: Mediators in Cell-to-Cell Interactions
To date, Gas6 and Pros1 are the most known TAM ligands, but other new potential ones have been described: tubby, tubby-like protein 1 [47], and galactin-3 (Gal-3) [48] seem to preferentially activate Mer during phagocytosis. However, little is still known regarding these new TAM ligands and this issue is beyond the scope of this review.
Both Gas6 and Pros1 are members of the vitamin K-dependent protein family: in fact, they contain a Gla domain in which the glutamate residues are posttranslationally modified to form gamma-carboxyglutamate through a vitamin K-dependent carboxylation. This latter reaction is required to confer to these proteins their activities. Moreover, Gas6 and Pros1 contain the SHBG-like domain that makes them unique compared to other vitamin K-dependent proteins: this domain shares 30% sequence identity with SHBG, it replaces the serine-protease domain found in other vitamin K-dependent plasma proteases [49], and it is devoid of enzymatic activity [50].
Pros1 circulates in plasma at a concentration of 346 nmol/L [51], and its expression can be found in several organs, such as the liver, kidney, lungs, and gonads [51], where it is produced by different cell types, like hepatocytes, endothelial cells, megakaryocytes, and osteoblasts [52]. Pros1 heterozygous deficiency is associated with an elevated risk of thrombosis development, whereas homozygous deficiency is lethal during embryonic development [51]. As stated above, Pros1, together with Gas6, is the most studied TAM ligand; it presents ~42% homology sequence with Gas6, and it specifically binds/activates Mer and Tyro3. Although Gas6 and Pros1 share structural homology, their functions are dissimilar, since the functions of Gas6 are limited to binding TAM. Instead, it is important to specify that Pros1 circulates in the bloodstream in two different forms: 60% of Pros1 is bound to the C4b-binding protein, while the remaining 40% of Pros1 is freely circulating [53]. Thus, only the “free Pros1” can bind and activate Mer and Tyro3. In addition, Pros1 contributes to the downregulation of thrombin formation by stimulating the activity as a nonenzymatic cofactor of both activated protein C and tissue factor pathway inhibitor [54, 55]. This latter essential function is TAM independent.
Gas6 interacts with TAM through its SHBG-like domain, positioned at the C-terminus of its sequence, activating downstream signaling pathways, such as PLCγ, PI3K, ERK, and NF-κB, and regulating cell survival, proliferation, migration, differentiation, adhesion, and apoptosis [56, 57].
Gas6 expression has been described in CD11b+F4/80+ bone marrow macrophages [58], in microglia [59], in peritoneal macrophages [14, 60], in apoptotic thymocytes [19], in Sertoli cells [61], and in CD11c+ dendritic cells of colon carcinoma [60]. Moreover, Gas6 is particularly expressed by endothelial cells, platelets, and leukocytes [62, 63].
Despite this, the biological role of Gas6 is not completely understood yet. Goruppi et al. showed that Gas6 is able to induce proliferation in vitro and to promote survival in the murine fibroblast cell line NIH-3T3 [64].
During the last years, different groups studying Gas6-TAM interaction focused on inflammation and tissue homeostasis, since in the presence of the Gla domain binding a PtdSer and the SHBG-like domain binding the Ig-like domain of TAM, Gas6 works as a bridge between apoptotic cells and the effector cells (Figure 1).
3. Gas6 and TAM Involvement in the Pathophysiology of Different Acute and Chronic Diseases
Gas6 and Pros1 are secreted in the bloodstream and, interestingly, Gas6 plasma levels in humans (~18 ng/mL) are two logarithms lower than Pros1 plasmatic ones [65]. Gas6 expression and its concentration in the bloodstream and in different compartments were found to change in several pathological conditions, both chronic and acute. These data allowed hypothesizing a role for Gas6 in the physiopathology of different diseases and using it as a tool for prognostic stratification in several specific contexts. For example, Bellan et al. demonstrated a correlation between plasmatic Gas6 levels and liver stiffness due to hepatic fibrosis from several etiologies [66]. In this context, they have introduced thresholds of plasmatic Gas6 for liver fibrosis (30 ng/mL) and severe fibrosis (42 ng/mL). Furthermore, the role of Gas6 as a predictor of esophageal varices was esteemed in patients affected by hepatitis C virus-related chronic liver disease [67]. In 2017, Staufer et al. strongly demonstrated the utility of sAxl and Gas6 serum levels as a diagnostic tool for advanced fibrosis, cirrhosis, and hepatocellular carcinoma on 392 patients, 361 of whom were affected by chronic liver disease from different etiologies. Moreover, they suggested the sAxl/albumin ratio as a better biomarker, since this ratio increases the accuracy to detect the degrees of these chronic liver diseases [68]. The use of Gas6 as a noninvasive biomarker has been proposed also by Li et al. in the early detection of diabetic nephropathy [69]. On the contrary, they observed decreased levels of Gas6 in diabetic patients suffering from the underestimated nephropathy and have proposed Gas6 (cutoff~9 ng/mL) as a better biomarker than cystatin C and creatinine. Concerning the renal pathophysiology, it has been shown that not only Gas6 but also sMer and sAxl have a potential role as biomarkers in patients affected by chronic kidney disease (CKD). Monocytes derived from CKD and hemodialysis patients showed a downregulation of Mer and Axl expression, both at RNA and plasma-membrane protein levels. However, plasmatic sMer and sAxl levels were remarkably higher in comparison to healthy subjects and they resulted to be positively associated with Gas6 levels in plasma of CKD patients [70].
Moreover, Sainaghi et al. found high Gas6 levels in the cerebrospinal fluid (CSF) of patients with a diagnosis of Alzheimer's disease (AD), with values that were doubled compared to the control group. The authors justified these findings as a compensatory mechanism: they hypothesized a Gas6 attempt to downregulate the proinflammatory cytokines, which are partially responsible for neuronal death [71]. Additionally, Gas6 has been found poorly expressed in the plasma of patients affected by multiple sclerosis, unlike sMer and sAxl [72]. However, Gas6 levels were found higher in CSF of these patients compared with control group, correlating with the relapse severity of the disease [73, 74]. Gas6 protein concentration in CSF was also found elevated in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) [75].
The Gas6 role as biomarker in SLE patients, particularly for those developing lupus nephritis and cutaneous vasculitis, suggested by Wu et al. in 2014 [76], has been recently confirmed by Gong et al. [77]. In addition, they showed an increase in the levels of soluble forms of Mer and Axl in these patients and they correlated the high levels of soluble receptors to proliferative glomerulonephritis.
However, the association between autoimmune diseases, SLE, and (s)TAM level/role is well established and reviewed elsewhere [24, 78].
Since TAM and their ligands have a wide range of functions and are expressed all over the body, it is reasonable to think of their possible involvement in acute diseases as well. It is reported that plasma Gas6 concentration is increased in patients with acute dyspnea due to heart failure and even more in patients with systemic or pulmonary infection [79]. Llacuna et al., for example, assumed a feasible therapeutic role of Gas6 after ischemia/reperfusion- (I/R-) induced hepatic injury in mice. They demonstrated that Gas6 homeostasis is regulated during I/R with its local release aimed at plugging the injury during the first phase; then, they observed a drastic decrease of Gas6 RNA during the reperfusion phase. Using mice knockout for Gas6 (Gas6−/−), the authors highlighted an increased susceptibility to hepatic I/R injury associated to enhanced expression of proinflammatory cytokines, such as IL-1β and tumor necrosis factor α (TNF-α), and increased levels of hepatic transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)). Moreover, they intravenously injected recombinant Gas6 (rGas6) in mice after hepatic I/R, in both Gas6 WT and Gas6−/− mice, observing that rGas6 injection not only rescued null mice from I/R-mediated liver injury but it also proved to be useful in protecting WT mice against hepatic I/R damage [80].
The therapeutic role of Gas6 has been suggested also by two other research groups using mouse models of sepsis-induced kidney injury [81] and sepsis-induced lung injury [82]. Chen et al. reported that intravenous injection of rGas6 immediately after sepsis induction exerts protective effects by reducing serum urea nitrogen, creatinine, and renal tissue apoptosis, thus attenuating the pathological damage and increasing the survival rate in a mouse model of sepsis-induced acute kidney injury following cecal ligation puncture (CLP) [81]. On the other hand, Giangola et al. reported that rGas6 administration behaves as an anti-inflammatory agent capable of abrogating sepsis-induced organ dysfunction and neutrophil-induced acute lung injury (ALI), resulting in the amelioration of the overall survival in a mouse model of CLP-induced sepsis [82].
4. An Open Window on Sepsis
Sepsis is one of the most common life-threatening diseases widespread in the world [83]. A crucial point concerning sepsis is to reach a fast diagnosis because of the multiple comorbidities and underlying diseases presented by septic patients [84].
The sepsis definition, in use until 2016, was based on the host's inflammatory responses. Recently, physicians and researchers have begun to break up the pathophysiology of sepsis discovering that the host reaction to sepsis involves not only the inflammatory milieu but also a modification in nonimmunological pathways [85]. This latest understanding led to a review of the sepsis definition and, in 2016, the Sepsis-3 conference defined sepsis as a “life-threatening organ dysfunction caused by a deregulated host response to infection” and septic shock as a “subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality” [86]. In this context, despite the presence of international recommendations [87], many points regarding the appropriate treatment still remain debatable [88–90]. As for the definition, diagnostic criteria have also changed and currently diagnosis is based on the detection of organ dysfunctions evaluated with the Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) score.
In the past, the SOFA score was created with the aim of calculating the number and severity of the dysfunction in six organ systems (notably pulmonary, coagulation, hepatobiliary, cardiovascular, renal, and neurologic) [91]. The Sepsis-3 definitions also introduced a new diagnostic tool useful in the early identification of patients at risk of sepsis in the emergency department (ED): the quik-SOFA (qSOFA) [92].
Over the last decade, there has been great interest in finding out biomarkers that could improve both sepsis diagnosis and sepsis prognosis [93–95]. In 2017, Kim et al. demonstrated a possible prognostic utility of procalcitonin (PCT), presepsin (sCD14-subtypes), soluble suppression of tumorigenicity 2 (sST2), and Gal-3 in sepsis.
They suggested that a multimarker approach could be beneficial for an optimized management of patients with sepsis [93]. The idea of a multimarker approach has been recently reclaimed by Mearelli et al. in a multicenter prospective study comprising a large cohort of patients. They developed and validated a high-performing, reproducible, and cost-effective algorithm to assist physicians of the emergency department in distinguishing sepsis/septic shock from noninfectious systemic inflammatory response syndrome (SIRS) [96]. Nowadays, it is becoming evident that the use of biomarkers in clinical procedures can be helpful and essential for a correct diagnosis, to discriminate noninfectious SIRS, sepsis, and septic shock patients, and to estimate the prognosis.
The abovementioned Gal-3 is one of the novel Mer ligands identified by Caberoy et al. They showed that Gal-3 stimulates the phagocytosis of apoptotic cells and cellular debris through Mer activation [48]. Since Gal-3 is involved in efferocytosis and it was found significantly higher in patients with sepsis and septic shock, Ferreira et al. induced sepsis in both WT and Gal-3 knockout mice showing that the absence of Gal-3 was protective against sepsis. This phenomenon seems to be associated with the ability of Gal-3 to limit neutrophil migration to primary sites of infection, consequently favoring bacterial spreading and death [97].
The employment of TAM and their ligands as biomarkers in septic patients has already been described more than ten years ago. Borgel et al.'s and Gibot et al.'s groups were among the first to depict the correlation between Gas6 and sepsis condition in 2006 and 2007, respectively [98, 99]. Few years later, Ekman et al. confirmed that Gas6 levels are increased during sepsis [100], finding a correlation between Gas6 and the degree of organ damage. In addition, they showed an increase of sAxl as well, although without the same magnitude of Gas6. Indeed, Gas6 levels strongly correlated with IL-6 and PCT levels and the number of failing organs. Thus, Gas6 levels were associated with disease severity and organ dysfunction. New studies have been conducted on a cohort of septic patients diagnosed following the Sepsis-3 criteria [101, 102]. In a cohort of 129 patients, it was reported that Gas6 plasmatic levels at admission in an intensive care unit (ICU) were higher in nonsurvivors than survivors [101]. However, neither Gas6 nor sAxl levels investigated in this study were able to discriminate bacteremic from nonbacteremic patients or Gram-negative versus Gram-positive infections. Moreover, Gas6 was compared with well-known inflammatory/severity biomarkers and evidence was found for a correlation between Gas6 levels and IL-6, IL-8, IL-10, sAxl, and PCT levels. Gas6 and IL-8 were the only two biomarkers found to be differently expressed between survivors and nonsurvivors. Therefore, these two biomarkers seem to be able to predict mortality in septic/shock patients at the time of ICU admission. In the same study, Gas6 performed better than procalcitonin and C-reactive protein, which are broadly used to diagnose infection, even though Gas6 levels between survivors and nonsurvivors remained constant over time. According to these findings, Gas6 cannot predict sepsis evolution, unlike other inflammatory mediators, such as TNF-α and IL-1β [101]. The role of Gas6 in septic patients was recently highlighted also in sepsis-related acute lung injury (ALI) by Yeh et al. [102]. Indeed, ALI is one of the complications of sepsis, and it is known for its contribution to sudden deaths and morbidity [103]. In this study published in 2017, the authors enrolled 129 patients with a diagnosis of sepsis and they compared the patients with and without ALI, observing that Gas6 levels, together with IL-6 and IL-8 levels, were significantly elevated among patients who developed ALI. Since nowadays a prompt and correct ALI diagnosis is mandatory in order to develop an effective treatment, the authors suggested Gas6 as an early predictor of ALI. Moreover, they suggested that Gas6 could also improve the parameters of the lung injury prediction score, such as its discrimination and its positive and negative predictive values [102].
The role of Gas6 in inflammatory contexts seems to be mainly related to its interaction with Mer [104, 105]. Mer has a pivotal role in counterbalancing the proinflammatory effects of toll-like receptor 4 (TRL4) activation induced by LPS, as demonstrated by Lee et al. using an anti-Mer neutralizing antibody [104].
Natural occurring regulatory T cells (Tregs) play a central role in maintaining immunologic homeostasis and tolerance. Different studies reported an expansion in both percentage and number of Tregs along with an increase in their suppressive function during sepsis [106]. Heuer et al. showed that adoptive transfer of in vitro-stimulated Tregs was able to increase the survival and the bacterial clearance in a mouse model of CLP-induced polymicrobial sepsis [107]. Zhao et al. demonstrated that Tregs express both Mer and Axl and that Gas6 administration in vivo increases forkhead box P3 (Foxp3) expression and suppressive activity by CD4+CD25+ Tregs. In vitro stimulation of Tregs by Gas6 had no effects on IL-10 and transforming growth factor β1 (TGF-β1) production, but it increased Foxp3 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression as well as the suppressive activity in a dose-dependent manner [108]. Hence, these studies suggest a possible role of Gas6 in tuning the immune response during sepsis by linking the innate and adaptive immune system.
However, the issue of comparing the response of the murine model of sepsis with human pathology is still open [109]. Regarding the focus of this review, we still know little about the response of TAM receptors and Gas6 in a murine sepsis model. Moreover, the levels of Gas6 and sAxl in both healthy and septic mice are not clear. Thus, the possibility that sAxl sequesters the endogenous circulating Gas6 is present in mice as well as in humans [45]. However, the administration of a large amount of exogenous Gas6 could overcome this problem by ameliorating the sepsis-induced multiorgan failure in septic mice, as recently demonstrated by Ni et al. [110]. Therefore, also in sepsis, where Gas6 levels are high, the injection of exogenous Gas6 seems to improve the outcome.
Summarizing, on the basis of previous studies, it is possible to hypothesize the use of Gas6 as a biomarker in the complex pathophysiology of sepsis, since several data seem to suggest a role of Gas6 as a useful biomarker for discriminating between noninfectious SIRS, sepsis, and septic shock. Furthermore, Gas6 came out as an early predictor of mortality and was able to identify some life-threatening sepsis complications. Moreover, Gas6 administration could be envisaged as a therapeutic reinforcement to the current treatment, since it showed to be able to ameliorate the overall survival and to partially protect from the organ dysfunction in a mouse model of sepsis. In conclusion, the Gas6/TAM axis activation possibly ameliorates the tissue hypoperfusion, thus restoring the physiological tissue homeostasis and preserving organ function, with a positive impact on sepsis prognosis (Figure 2).
Figure 2.
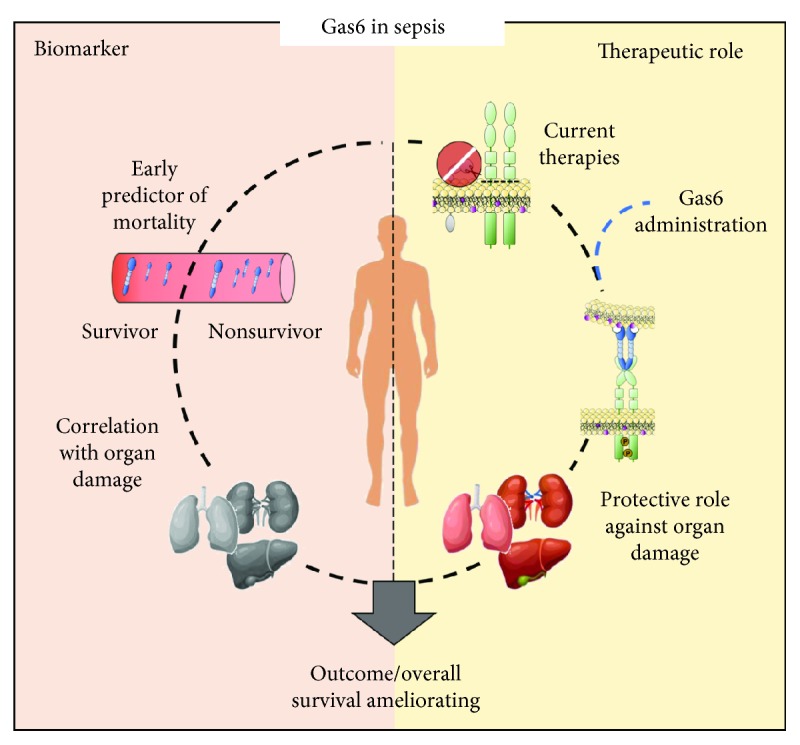
Gas6: the paradoxical role in sepsis. During sepsis, Gas6 could be used as an early biomarker in the routine management of septic patients since Gas6 plasma levels, measured at the time of ICU admission, can predict mortality and multiorgan failure. The high levels of Gas6 released in the bloodstream during sepsis seem to be aimed at counterbalancing sepsis dysfunctions; however, because inflammatory stimuli downregulate TAM receptors, the Gas6 overrelease is ineffective. Current therapy for sepsis is aimed at decreasing inflammatory stimuli. Gas6 administration after current therapy could operate on activated TAM receptors and protect the organs from sepsis-induced damage. The combination of a correct early diagnosis and the protective effects mediated by Gas6 could ameliorate the outcome/overall survival of patients.
Abbreviations
- Gas6:
Growth arrest-specific 6
- RTKs:
Tyrosine kinase receptors
- DCs:
Dendritic cells
- BM:
Bone marrow
- HSCs:
Hepatic stellate cells
- LSECs:
Liver sinusoidal endothelial cells
- NK:
Natural killer
- NKT:
Natural killer T
- Pros1:
Protein S
- SHGB:
Sex hormone-binding globulin
- Gla:
Gamma-carboxyglutamic acid rich
- PtdSer:
Phosphatidylserine
- PI3K:
Phosphoinositide 3 kinase
- MAP:
Mitogen-activated protein
- NF-κB:
Nuclear factor-light-chain-enhancer of activated B cells
- STAT:
Signal transducer and activator of transcription protein
- PLC:
Phospholipase C
- Grb2:
Growth factor receptor-bound protein 2
- ERK:
Extracellular-signal-regulated kinase
- SOCS:
Suppressor of cytokine signaling proteins
- TKO:
Triple knockout
- WT:
Wild type
- SLE:
Systemic lupus erythematosus
- APCs:
Antigen-presenting cells
- IL:
Interleukin
- ADAM17:
A disintegrin and metalloproteinase domain 17
- TACE:
Tumor necrosis factor-alpha converting enzyme
- PRRs:
Pattern-recognition receptors
- LPS:
Lipopolysaccharide
- Poly:C:
Polycytidylic acid
- PolyI:C:
Polyinosinic:polycytidylic acid
- sAxl:
Soluble Axl
- sMer:
Soluble Mer
- TK:
Tyrosine kinase
- FN III:
Fibronectin type III domains
- CKD:
Chronic kidney disease
- CSF:
Cerebrospinal fluid
- AD:
Alzheimer's disease
- CIDP:
Chronic inflammatory demyelinating polyneuropathy
- I/R:
Ischemia/reperfusion
- TNF-α:
Tumor necrosis factor α
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- rGas6:
Recombinant Gas6
- CLP:
Cecal ligation puncture
- SOFA:
Sequential (sepsis-related) organ failure assessment
- ED:
Emergency department
- qSOFA:
Quick SOFA
- PCT:
Procalcitonin
- Gal-3:
Galectin-3
- sST2:
Soluble suppression of tumorigenicity 2
- SIRS:
Systemic inflammatory response syndrome
- ALI:
Acute lung injury
- TLR4:
Toll-like receptor 4
- TSLP:
Thymic stromal lymphopoietin
- TSLPR:
TSLP receptor
- CCL17:
Chemokine (C-C motif) ligand 17
- Tregs:
Regulatory T cells
- Foxp3:
Forkhead box P3.
Conflicts of Interest
All the authors state they have no conflicts of interest.
References
- 1.Lemmon M. A., Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothlin C. V., Carrera-Silva E. A., Bosurgi L., Ghosh S. TAM receptor signaling in immune homeostasis. Annual Review of Immunology. 2015;33(1):355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zagórska A., Través P. G., Lew E. D., Dransfield I., Lemke G. Diversification of TAM receptor tyrosine kinase function. Nature Immunology. 2014;15(10):920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji R., Meng L., Jiang X., et al. TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan E., Yeap X. Y., Dehn S., et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circulation Research. 2013;113(8):1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandahl M., Hunter D. M., Strunk K. E., Earp H. S., Cook R. S. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Developmental Biology. 2010;10(1):p. 122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimori T., Grabiec A. M., Kaur M., et al. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunology. 2015;8(5):1021–1030. doi: 10.1038/mi.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazeros A., Harvey B. G., Carolan B. J., Vanni H., Krause A., Crystal R. G. Overexpression of apoptotic cell removal receptor MERTK in alveolar macrophages of cigarette smokers. American Journal of Respiratory Cell and Molecular Biology. 2008;39(6):747–757. doi: 10.1165/rcmb.2007-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee S. K., Wilhelm A., Antoniades C. G. TAM receptor tyrosine kinase function and the immunopathology of liver disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2016;310(11):G899–G905. doi: 10.1152/ajpgi.00382.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian M., Hayes C. D., Thome J. J., et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. Journal of Clinical Investigation. 2014;124(3):1296–1308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao W.-H., Zhen Y., Eisenberg R. A., Cohen P. L. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clinical Immunology. 2009;133(1):138–144. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong F., Chen Z., Zhang L., et al. Tyro3 is a podocyte protective factor in glomerular disease. JCI Insight. 2018;3(22) doi: 10.1172/jci.insight.123482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Chen Y., Ge Y., et al. Immunoexpression of Tyro 3 family receptors—Tyro 3, Axl, and Mer—and their ligand Gas6 in postnatal developing mouse testis. Journal of Histochemistry & Cytochemistry. 2005;53(11):1355–1364. doi: 10.1369/jhc.5A6637.2005. [DOI] [PubMed] [Google Scholar]
- 14.Deng T., Zhang Y., Chen Q., Yan K., Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135(1):40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould W. R., Baxi S. M., Schroeder R., et al. Gas6 receptors Axl, Sky and Mer enhance platelet activation and regulate thrombotic responses. Journal of Thrombosis and Haemostasis. 2005;3(4):733–741. doi: 10.1111/j.1538-7836.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 16.Malawista A., Wang X., Trentalange M., Allore H. G., Montgomery R. R. Coordinated expression of tyro3, axl, and mer receptors in macrophage ontogeny. Macrophage. 2016;3 doi: 10.14800/macrophage.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paolino M., Choidas A., Wallner S., et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitz H. M., Camenisch T. D., Lemke G., Earp H. S., Matsushima G. K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. The Journal of Immunology. 2007;178(9):5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 19.Wallet M. A., Sen P., Flores R. R., et al. MerTK is required for apoptotic cell-induced T cell tolerance. The Journal of Experimental Medicine. 2008;205(1):219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens E. . M., Gadue P., Gong S.-y., Garrett S., Stein P. . L., Cohen P. . L. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. European Journal of Immunology. 2003;33(8):2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- 21.Scott R. S., McMahon E. J., Pop S. M., et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 22.Wu G., Ma Z., Cheng Y., et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Molecular Cancer. 2018;17(1):p. 20. doi: 10.1186/s12943-018-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce A. M., Keating A. K. TAM receptor tyrosine kinases: expression, disease and oncogenesis in the central nervous system. Brain Research. 2014;1542:206–220. doi: 10.1016/j.brainres.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wium M., Paccez J., Zerbini L. The dual role of TAM receptors in autoimmune diseases and cancer: an overview. Cells. 2018;7(10):p. 166. doi: 10.3390/cells7100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew E. D., Oh J., Burrola P. G., et al. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. eLife. 2014;3 doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alciato F., Sainaghi P. P., Sola D., Castello L., Avanzi G. C. TNF-α, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. Journal of Leukocyte Biology. 2010;87(5):869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 27.Korshunov V. A. Axl-dependent signalling: a clinical update. Clinical Science. 2012;122(8):361–368. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemke G. Biology of the TAM receptors. Cold Spring Harbor Perspectives in Biology. 2013;5(11) doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothlin C. V., Ghosh S., Zuniga E. I., Oldstone M. B. A., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Akalu Y. T., Rothlin C. V., Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunological Reviews. 2017;276(1):165–177. doi: 10.1111/imr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parinot C., Nandrot E. F. A comprehensive review of mutations in the MERTK proto-oncogene. Retinal Degenerative Diseases. 2016;854 doi: 10.1007/978-3-319-17121-0_35. [DOI] [PubMed] [Google Scholar]
- 32.Lemke G., Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Annals of the New York Academy of Sciences. 2010;1209(1):23–29. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T. Biological roles of hepatocyte growth factor‑Met signaling from genetically modified animals (Review) Biomedical Reports. 2017;7 doi: 10.3892/br.2017.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Wang H., Qi N., et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138(4):655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 35.Prasad D., Rothlin C. V., Burrola P., et al. TAM receptor function in the retinal pigment epithelium. Molecular and Cellular Neuroscience. 2006;33(1):96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Duncan J. L., LaVail M. M., Yasumura D., et al. An RCS-Like Retinal Dystrophy Phenotype inMerKnockout Mice. Investigative Opthalmology & Visual Science. 2003;44(2):p. 826. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 37.Lu Q. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 38.Qi N., Liu P., Zhang Y., Wu H., Chen Y., Han D. Development of a spontaneous liver disease resembling autoimmune hepatitis in mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. PLoS One. 2013;8(6):p. e66604. doi: 10.1371/journal.pone.0066604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorp E., Vaisar T., Subramanian M., Mautner L., Blobel C., Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK) Journal of Biological Chemistry. 2011;286(38):33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller M. A., Sullivan R. J., Lauffenburger D. A. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clinical Cancer Research. 2017;23(3):623–629. doi: 10.1158/1078-0432.CCR-16-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J., Ekman C., Jönsen A., et al. Increased plasma levels of the soluble Mer tyrosine kinase receptor in systemic lupus erythematosus relate to disease activity and nephritis. Arthritis Research & Therapy. 2011;13(2):p. R62. doi: 10.1186/ar3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L., Hu F., Zhu H., et al. Soluble TAM receptor tyrosine kinases in rheumatoid arthritis: correlation with disease activity and bone destruction. Clinical & Experimental Immunology. 2018;192(1):95–103. doi: 10.1111/cei.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sather S., Kenyon K. D., Lefkowitz J. B., et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2006;109(3):1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orme J. J., du Y., Vanarsa K., et al. Heightened cleavage of Axl receptor tyrosine kinase by ADAM metalloproteases may contribute to disease pathogenesis in SLE. Clinical Immunology. 2016;169:58–68. doi: 10.1016/j.clim.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekman C., Stenhoff J., Dahlbäck B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. Journal of Thrombosis and Haemostasis. 2010;8(4):838–844. doi: 10.1111/j.1538-7836.2010.03752.x. [DOI] [PubMed] [Google Scholar]
- 46.Nagata K., Ohashi K., Nakano T., et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. Journal of Biological Chemistry. 1996;271(47):30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 47.Caberoy N. B., Zhou Y., Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. The EMBO Journal. 2010;29(23):3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caberoy N. B., Alvarado G., Bigcas J.-L., Li W. Galectin-3 is a new MerTK-specific eat-me signal. Journal of Cellular Physiology. 2012;227(2):401–407. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker M. E., French F. S., Joseph D. R. Vitamin K-dependent protein S is similar to rat androgen-binding protein. Biochemical Journal. 1987;243(1):293–296. doi: 10.1042/bj2430293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saposnik B., Borgel D., Aiach M., Gandrille S. Functional properties of the sex-hormone-binding globulin (SHBG)-like domain of the anticoagulant protein S. European Journal of Biochemistry. 2003;270(3):545–555. doi: 10.1046/j.1432-1033.2003.03423.x. [DOI] [PubMed] [Google Scholar]
- 51.van der Meer J. H. M., van der Poll T., van 't Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood. 2014;123(16):2460–2469. doi: 10.1182/blood-2013-09-528752. [DOI] [PubMed] [Google Scholar]
- 52.Maillard C., Berruyer M., Serre C. M., Dechavanne M., Delmas P. D. Protein-S, a vitamin K-dependent protein, is a bone matrix component synthesized and secreted by osteoblasts. Endocrinology. 1992;130(3):1599–1604. doi: 10.1210/endo.130.3.1531628. [DOI] [PubMed] [Google Scholar]
- 53.Dahlbäck B. The tale of protein S and C4b-binding protein, a story of affection. Thrombosis and Haemostasis. 2007;98(07):90–96. doi: 10.1160/th07-04-0269. [DOI] [PubMed] [Google Scholar]
- 54.Walker F. J. Protein S and the regulation of activated protein C. Seminars in Thrombosis and Hemostasis. 1984;10(02):131–138. doi: 10.1055/s-2007-1004415. [DOI] [PubMed] [Google Scholar]
- 55.Robins R. S., Lemarié C. A., Laurance S., Aghourian M. N., Wu J., Blostein M. D. Vascular Gas6 contributes to thrombogenesis and promotes tissue factor up-regulation after vessel injury in mice. Blood. 2013;121(4):692–699. doi: 10.1182/blood-2012-05-433730. [DOI] [PubMed] [Google Scholar]
- 56.Axelrod H., Pienta K. J. Axl as a mediator of cellular growth and survival. Oncotarget. 2014;5(19):8818–8852. doi: 10.18632/oncotarget.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu K.-S., Hung Y.-J., Lee C.-H., Hsiao F.-C., Hsieh P.-S. The involvement of GAS6 signaling in the development of obesity and associated inflammation. International Journal of Endocrinology. 2015;2015:7. doi: 10.1155/2015/202513. 2015.202513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zahuczky G., Kristóf E., Majai G., Fésüs L. Differentiation and glucocorticoid regulated apopto-phagocytic gene expression patterns in human macrophages. Role of Mertk in enhanced phagocytosis. PLoS one. 2011;6(6):p. e21349. doi: 10.1371/journal.pone.0021349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butovsky O., Jedrychowski M. P., Moore C. S., et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nature Neuroscience. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loges S., Schmidt T., Tjwa M., et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115(11):2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 61.Lu Q., Gore M., Zhang Q., et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 62.Manfioletti G., Brancolini C., Avanzi G., Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Molecular and Cellular Biology. 1993;13(8):4976–4985. doi: 10.1128/MCB.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avanzi G. C., Gallicchio M., Cavalloni G., et al. GAS6, the ligand of Axl and Rse receptors, is expressed in hematopoietic tissue but lacks mitogenic activity. Experimental Hematology. 1997;25(12):1219–1226. [PubMed] [Google Scholar]
- 64.Goruppi S., Ruaro E., Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12(3):471–480. [PubMed] [Google Scholar]
- 65.Balogh I., Hafizi S., Stenhoff J., Hansson K., Dahlbäck B̈. Analysis of Gas6 in human platelets and plasma. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1280–1286. doi: 10.1161/01.ATV.0000163845.07146.48. [DOI] [PubMed] [Google Scholar]
- 66.Bellan M., Pogliani G., Marconi C., et al. Gas6 as a putative noninvasive biomarker of hepatic fibrosis. Biomarkers in Medicine. 2016;10(12):1241–1249. doi: 10.2217/bmm-2016-0210. [DOI] [PubMed] [Google Scholar]
- 67.Bellan M., Sainaghi P. P., Minh M. T., et al. Gas6 as a predictor of esophageal varices in patients affected by hepatitis C virus related-chronic liver disease. Biomarkers in Medicine. 2018;12(1):27–34. doi: 10.2217/bmm-2017-0171. [DOI] [PubMed] [Google Scholar]
- 68.Staufer K., Dengler M., Huber H., et al. The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death & Disease. 2017;8(10):p. e3135. doi: 10.1038/cddis.2017.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W., Wang J., Ge L., Shan J., Zhang C., Liu J. Growth arrest-specific protein 6 (Gas6) as a noninvasive biomarker for early detection of diabetic nephropathy. Clinical and Experimental Hypertension. 2017;39(4):382–387. doi: 10.1080/10641963.2017.1288739. [DOI] [PubMed] [Google Scholar]
- 70.Lee I. J., Hilliard B. A., Ulas M., et al. Monocyte and plasma expression of TAM ligand and receptor in renal failure: links to unregulated immunity and chronic inflammation. Clinical Immunology. 2015;158(2):231–241. doi: 10.1016/j.clim.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Sainaghi P. P., Bellan M., Lombino F., et al. Growth arrest specific 6 concentration is increased in the cerebrospinal fluid of patients with Alzheimer’s disease. Journal of Alzheimer's Disease. 2016;55(1):59–65. doi: 10.3233/JAD-160599. [DOI] [PubMed] [Google Scholar]
- 72.Weinger J. G., Omari K. M., Marsden K., Raine C. S., Shafit-Zagardo B. Up-regulation of soluble Axl and Mer receptor tyrosine kinases negatively correlates with Gas6 in established multiple sclerosis lesions. The American Journal of Pathology. 2009;175(1):283–293. doi: 10.2353/ajpath.2009.080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sainaghi P. P., Collimedaglia L., Alciato F., et al. Growth arrest specific gene 6 protein concentration in cerebrospinal fluid correlates with relapse severity in multiple sclerosis. Mediators of Inflammation. 2013;2013:7. doi: 10.1155/2013/406483.406483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellan M., Pirisi M., Sainaghi P. P. The Gas6/TAM system and multiple sclerosis. International Journal of Molecular Sciences. 2016;17(11):p. 1807. doi: 10.3390/ijms17111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sainaghi P. P., Collimedaglia L., Alciato F., et al. Elevation of Gas6 protein concentration in cerebrospinal fluid of patients with chronic inflammatory demyelinating polyneuropathy (CIDP) Journal of the Neurological Sciences. 2008;269(1-2):138–142. doi: 10.1016/j.jns.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Wu C. S., Hu C. Y., Tsai H. F., et al. Elevated serum level of growth arrest-specific protein 6 (Gas6) in systemic lupus erythematosus patients is associated with nephritis and cutaneous vasculitis. Rheumatology International. 2014;34(5):625–629. doi: 10.1007/s00296-013-2882-1. [DOI] [PubMed] [Google Scholar]
- 77.Gong S., Xu Z., Liu Y., et al. Plasma sMer, sAxl and GAS6 levels correlate with disease activity and severity in lupus nephritis. European Journal of Clinical Investigation. 2019;49(3):p. e13064. doi: 10.1111/eci.13064. [DOI] [PubMed] [Google Scholar]
- 78.Rothlin C. V., Lemke G. TAM receptor signaling and autoimmune disease. Current Opinion in Immunology. 2010;22(6):740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sainaghi P. P., Alciato F., Carnieletto S., et al. Gas6 evaluation in patients with acute dyspnea due to suspected pulmonary embolism. Respiratory Medicine. 2009;103(4):589–594. doi: 10.1016/j.rmed.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 80.Llacuna L., Bárcena C., Bellido-Martín L., et al. Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology. 2010;52(4):1371–1379. doi: 10.1002/hep.23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L. W., Chen W., Hu Z. Q., et al. Protective effects of growth arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney injury. Inflammation. 2016;39(2):575–582. doi: 10.1007/s10753-015-0282-2. [DOI] [PubMed] [Google Scholar]
- 82.Giangola M. D., Yang W.-L., Rajayer S. R., Nicastro J., Coppa G. F., Wang P. Growth arrest-specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock. 2013;40(6):485–491. doi: 10.1097/SHK.0b013e3182a588c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudd K. E., Kissoon N., Limmathurotsakul D., et al. The global burden of sepsis: barriers and potential solutions. Critical Care. 2018;22(1):p. 232. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novosad S. A., Sapiano M. R. P., Grigg C., et al. Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. MMWR. Morbidity and Mortality Weekly Report. 2016;65(33):864–869. doi: 10.15585/mmwr.mm6533e1. [DOI] [PubMed] [Google Scholar]
- 85.Rello J., Valenzuela-Sánchez F., Ruiz-Rodriguez M., Moyano S. Sepsis: a review of advances in management. Advances in Therapy. 2017;34(11):2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singer M., Deutschman C. S., Seymour C. W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rhodes A., Evans L. E., Alhazzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 88.Jozwiak M., Hamzaoui O., Monnet X., Teboul J.-L. Fluid resuscitation during early sepsis: a need for individualization. Minerva Anestesiologica. 2018;84(8):987–992. doi: 10.23736/S0375-9393.18.12422-9. [DOI] [PubMed] [Google Scholar]
- 89.Beurton A., Teboul J. L., Gavelli F., et al. The effects of passive leg raising may be detected by the plethysmographic oxygen saturation signal in critically ill patients. Critical Care. 2019;23(1) doi: 10.1186/s13054-019-2306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy M. M., Evans L. E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Medicine. 2018;44(6):925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 91.Vincent J. L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 92.Seymour C. W., Liu V. X., Iwashyna T. J., et al. Assessment of clinical criteria for sepsis. JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.on behalf of GREAT Network, Kim H., Hur M., Moon H.-W., Yun Y.-M., Di Somma S. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. 2017;7(1):p. 27. doi: 10.1186/s13613-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.AdrenOSS-1 study investigators, Mebazaa A., Geven C., et al. Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study. Critical Care. 2018;22(1) doi: 10.1186/s13054-018-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castello L. M., Baldrighi M., Molinari L., et al. The role of osteopontin as a diagnostic and prognostic biomarker in sepsis and septic shock. Cells. 2019;8(2):p. 174. doi: 10.3390/cells8020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mearelli F., Fiotti N., Giansante C., et al. Derivation and validation of a biomarker-based clinical algorithm to rule out sepsis from noninfectious systemic inflammatory response syndrome at emergency department admission. Critical Care Medicine. 2018;46(9):1421–1429. doi: 10.1097/CCM.0000000000003206. [DOI] [PubMed] [Google Scholar]
- 97.Ferreira R. G., Rodrigues L. C., Nascimento D. C., et al. Galectin-3 aggravates experimental polymicrobial sepsis by impairing neutrophil recruitment to the infectious focus. Journal of Infection. 2018;77(5):391–397. doi: 10.1016/j.jinf.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Borgel D., Clauser S., Bornstain C., et al. Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Critical Care Medicine. 2006;34(1):219–222. doi: 10.1097/01.CCM.0000195014.56254.8A. [DOI] [PubMed] [Google Scholar]
- 99.Gibot S., Massin F., Cravoisy A., et al. Growth arrest-specific protein 6 plasma concentrations during septic shock. Critical Care. 2007;11(1):p. R8. doi: 10.1186/cc5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ekman C., Linder A., Akesson P., Dahlbäck B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Critical Care. 2010;14(4):p. R158. doi: 10.1186/cc9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stalder G., Que Y. A., Calzavarini S., et al. Study of early elevated Gas6 plasma level as a predictor of mortality in a prospective cohort of patients with sepsis. PLoS One. 2016;11(10):p. e0163542. doi: 10.1371/journal.pone.0163542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeh L.-C., Huang P. W., Hsieh K. H., et al. Elevated plasma levels of Gas6 are associated with acute lung injury in patients with severe sepsis. The Tohoku Journal of Experimental Medicine. 2017;243(3):187–193. doi: 10.1620/tjem.243.187. [DOI] [PubMed] [Google Scholar]
- 103.Fujishima S., Gando S., Daizoh S., et al. Infection site is predictive of outcome in acute lung injury associated with severe sepsis and septic shock. Respirology. 2016;21(5):898–904. doi: 10.1111/resp.12769. [DOI] [PubMed] [Google Scholar]
- 104.Lee Y.-J., Han J. Y., Byun J., et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-κB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. Journal of Leukocyte Biology. 2012;91(6):921–932. doi: 10.1189/jlb.0611289. [DOI] [PubMed] [Google Scholar]
- 105.Choi J.-Y., Seo J. Y., Yoon Y.-S., Lee Y.-J., Kim H.-S., Kang J. L. Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Science Signaling. 2015;8(365):p. ra21. doi: 10.1126/scisignal.2005864. [DOI] [PubMed] [Google Scholar]
- 106.Cao C., Ma T., Chai Y.-F., Shou S.-T. The role of regulatory T cells in immune dysfunction during sepsis. World Journal of Emergency Medicine. 2015;6(1):p. 5. doi: 10.5847/wjem.j.1920-8642.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heuer J. G., Zhang T., Zhao J., et al. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. The Journal of Immunology. 2005;174(11):7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 108.Zhao G.-J., Zheng J. Y., Bian J. L., et al. Growth arrest-specific 6 enhances the suppressive function of CD4+CD25+ regulatory T cells mainly through Axl receptor. Mediators of Inflammation. 2017;2017:13. doi: 10.1155/2017/6848430.6848430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lewis A. J., Seymour C. W., Rosengart M. R. Current murine models of sepsis. Surgical Infections. 2016;17(4):385–393. doi: 10.1089/sur.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ni J., Lin M., Jin Y., et al. Gas6 attenuates sepsis-induced tight junction injury and vascular endothelial hyperpermeability via the Axl/NF-κB signaling pathway. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]