Abstract
Background:
Gentamicin-induced-acute kidney injury (AKI) is a multifaceted phenomenon which previously linked to the oxidative stress only. Vinpocetine prevents reactive free radical generation which contributed in reduction of damage. Therefore, objective of the present study was to investigate the renoprotective effect of vinpocetine on gentamicin-induced-AKI in rats.
Methods:
Thirty Sprague Dawley Male rat were divided into three groups. Control group (n = 10): Rats treated with distilled water + intra-peritoneal injection of normal saline 2 ml/kg/day. Gentamicin group (n = 10): Rats treated with distilled water + intra-peritoneal injection of gentamicin 100 mg/kg/day. Vinpocetine group (n = 10): Rats treated with vinpocetine + intra-peritoneal injection of gentamicin 100 mg/kg/day. Blood urea and serum creatinine were estimated by auto-analyzer. Serum malondialdehyde (MDA), superoxide dismutase (SOD), Neutrophil Gelatinase Associated Lipocalin (NGAL), kidney injury molecules (KIM-1), and Cystatin-c were measured by ELISA kit methods.
Results:
Vinpocetine led to significant renoprotective effect on gentamicin induced-AKI through amelioration of blood urea and serum creatinine compared with gentamicin group P < 0.01. Vinpocetine improved oxidative stress through reduction of MDA serum level and elevation of SOD significantly compared with gentamicin group P = 0.001 and P = 0.03, respectively. Indeed, vinpocetine reduced glomerular and renal tubular injury via reduction of inflammatory biomarkers including KIM-1, NGALand Cystatin-c sera levels significantly P < 0.01 compared to gentamicin group.
Conclusions:
Vinpocetine leads to significant attenuation of gentamicin-induced-AKI through modulation of oxidative stress and pro-inflammatory pathway.
Keywords: AKI, gentamicins, oxidative stresses, pro-inflammatory pathways, vinpocetines
Introduction
Gentamicin is a bactericidal antibiotic belongs to the aminoglycoside group used for treatment of different bacterial infections, mainly for of urinary tract infection, pneumonia, bone infection, meningitis, and pelvic inflammatory diseases. Gentamicin consists of a different numbers of related compounds including gentamicin C1, C1a, and C2 (which have 80% of antibacterial activity) while; gentamicin A, B, X (have 20% of antibacterial activity) [Figure 1].[1]
Figure 1.
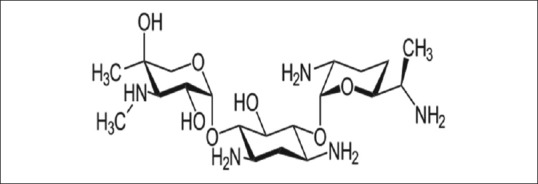
Chemical structure of gentamicin
Gentamicin-induced-acute kidney injury (AKI) is a multifaceted phenomenon which previously linked to the oxidative stress only. Different studies and current researches illustrated more complex mechanisms related to this type of toxicity.[2]
Gentamicin extensively and selectively deposits in the epithelial cells of proximal renal tubules leading to lysosomal aggregation, alteration phospholipid and lipid metabolism.[3]
The selective accumulation of gentamicin in the proximal renal tubules is due to presence of megalin and cubilin, which are membrane endocytic proteins that are involved in the transport of cations such as aminoglycosides and xenobiotics. This accumulation alters the function of epithelial cells of proximal renal tubules via alteration of extra-cellular calcium sensing receptors leading to necrosis and cell death.[4]
Small amounts of gentamicin enter the nucleus independent on cubiline/megalin complex, as well it enters the cytosol through transient receptor potential vanilloid type 4 channel which is expressed only in the distal renal tubule due to cytotoxicity. These changes lead to rupture of lysosome and release of endogenous cathepsins causing proteolysis and necrosis of renal tubular cells. Additionally, gentamicin may cause direct effect through induction the liberation of pro-apoptotic mediators that cause significant cell death and AKI.[5]
Chronic high dose of gentamicin initiates in vitro and in vivo free radical productions and induction of oxidative stress. Gentamicin activates mitochondrial superoxide anions causing generation of hydrogen peroxide and hydroxyl radicals. Besides, gentamicin stimulates mitochondrial respiratory chain for generations of free radicals.[6] Previous studies documented that gentamicin therapy led to significant reduction in the activity of glutathione, superoxide dismutase, and other endogenous anti-oxidant capacity at renal proximal tubular cells which contributed in part in gentamicin-induced-AKI.[7] Therefore, anti-oxidant agents afford renoprotective effect and reduce AKI via reduction of tissue damage and oxidative stress.[8]
Vinpocetine is an alkaloid vincamine derivative (a synthetic ethyl ester of apovincamine) used in treatment of cognitive disorders and cerebrovascular diseases [Figure 2].[9]
Figure 2.
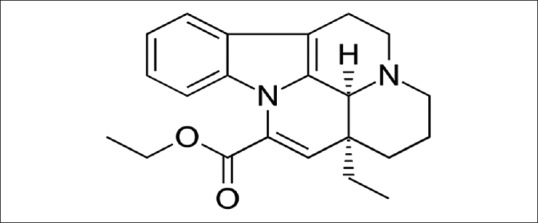
Chemical structure of vinpocetine
Vinpocetine improves cerebral metabolism through increasing of cerebral blood flow and augmentation of oxygen consumption. It is specific phosphodiestrase enzyme (PDE) type 1 inhibitor which reduces blood viscosity and improves peripheral circulation.[10]
The chief anti-inflammatory and anti-oxidant mechanisms of vinpocetine are through inhibition of enzyme complex involved in accentuation of cellular response to inflammation, such as IkB kinase enzyme, inhibition of NF-kB (protein complex controls DNA transcription, cell survival, and cytokine productions). Besides, vinpocetine prevents reactive free radical generation which contributed into reduction of high glucose-induced-oxidative damage.[11]
Therefore, objective of the present study was to investigate the renoprotective effect of vinpocetine in gentamicin-induced-AKI in rats.
Methods
Thirty Sprague Dawley Male rat were used, which were gained from the National Center for Drug Control and Research, aged 2–3 months and weighing 100–200 g. They were kept at a suitable room temperature and artificial 12/12 light-dark cycle with free access to normal chow pellets and water. This experimental study was approved by Ethical Committee in College of Medicine by Scientific Board according to Humane Care for Animals and Guide to the care and use of laboratory animal. The drugs were purchased from private pharmaceutical company, Gentamicin ampoule (Garamycin 80 mg Schering-Plough, USA) and vinpocetine tablet (neurovin 5 mg MICRO LAPS LIMITED, India).
After an acclimatization period of 1 week, rats were randomly divided into three groups, 10 rats in each group. The study protocol and method for induction of AKI was according to Ortega et al., method.[12]
Control group (n = 10): Rats treated with distilled water (2 ml/kg/day) orally for 10 days and on 5th day they received intra-peritoneal injection of normal saline 2 ml/kg/day.
Gentamicin group (n = 10): Rats treated with distilled water (2 ml/kg/day) orally for 10 days and on 5th day they received intra-peritoneal injection of gentamicin 100 mg/kg/day.
Vinpocetine group (n = 10): Rats treated with vinpocetine (5 mg/kg/day) orally for 10 days and on the 5th day they received intra-peritoneal injection of gentamicin 100 mg/kg/day. (Vinpocetin tablet was dissolved in 5 mL of 0.9% normal saline).
Sample collection
On the end of 10th day, rat decapitation was done under chloroform anesthesia. The blood samples were centrifuged at 5000 rpm/5 minute and all sera were kept at -20 for later assessment.
Assessment the biomarkers of AKI
Blood urea and serum creatinine were estimated by using auto-analyzer (ILab-300-Biomerieux Diagnostic, Milano, Italy) they expressed as mg/dL).
Serum malondialdehyde (MDA), superoxide dismutase (SOD), Neutrophil Gelatinase Associated Lipocalin (NGAL), kidney injury molecules (KIM-1), and Cystatin-c were measured by ELISA kit methods according to the instruction of the kit manufacture (Myo-bio source, USA).
Assessment of glomerular filtration rate (GFR)
Creatinine based-estimated GFR
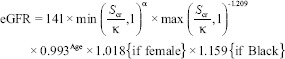
Scr = serum creatinine in mgldl, k = 0.7 for female 0.9 for male, α = −0.329 for female −0.411 for male, max = maximum, mim = minium, age in years.[13]
Normal = eGFR >90 ml/min/1.73 m2
Mild = eGFR (60-89) ml/min/1.73 m2
Moderate = (30-59) ml/min/1.73 m2
Severe = (15 = 29) ml/min/1.73 m2
Acute renal failure = eGFR < 15 ml/min/1.72 m2
Cystatin based-estimated GFR
GFR-Cys = 162/cyst (mg/l)-30.[14]
Statistical analysis
Data of the present study presented as mean ± SD. One-way ANOVA test with Bonferroni post-hoc test was used to investigate the significance of differences among different groups. Statistical package for the Social Sciences Software (SPSS Inc., Chicago, IL, USA) was used for data analysis. The levels of significance were considered when P < 0.05.
Results
Changes in the biochemical and inflammatory biomarkers in gentamicin-induced-AKI
In gentamicin-induced-AKI, blood urea was increased up to 58.87 ± 6.45 mg/dL compared with 40.83 ± 5.46 mg/dL in the control group P = 0.001 as well; serum creatinine was increased in gentamicin group up to 2.08 ± 0.20 mg/dL compared with the control group 0.70 ± 0.14 mg/dL P = 0.0004. MDA serum level was increased in gentamicin group to 398.11 ± 14.8 (ng/mL) compared with 290.85 ± 10.18 (ng/mL) in control group P = 0.001. But SOD serum level was reduced in gentamicin group to 11.89 ± 2.94 (pg/mL) compared with 16.94 ± 2.39 (pg/mL) in control group P = 0.0009. Moreover, KIM-1, NGAL, and the Cyst-c sera levels were significantly elevated in gentamicin group compared with the control group P < 0.01 Table 1.
Table 1.
Effect of vinpocetine on the biochemical and inflammatory biomarkers in gentamicin-induced-AKI
| Variables | Control (n=10) | G+S (n=10) | G+V (n=10) | Post-hoc test | ANOVA | ||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| Blood urea (mg/dL) | 40.83±5.46 | 58.87±6.45 | 42.56±5.89 | 0.001 | NS | 0.001 | 0.0001 |
| Serum cr.(mg/dL) | 0.70±0.14 | 2.08±0.20 | 1.32±0.22 | 0.0004 | 0.001 | 0.001 | 0.0005 |
| MDA (ng/mL) | 290.85±10.18 | 398.11±14.8 | 298.52±10.87 | 0.001 | NS | 0.001 | 0.0001 |
| SOD (pg/mL) | 16.94±2.39 | 11.89±2.94 | 14.77±2.86 | 0.0009 | NS | 0.03 | 0.001 |
| KIM-1(pg/mL) | 79.78±9.29 | 99.98±10.38 | 83.56±9.98 | 0.001 | NS | 0.001 | 0.0002 |
| NGAL (pg/mL) | 15.78±3.07 | 24.04±5.88 | 16.67±3.98 | 0.0009 | NS | 0.002 | 0.0005 |
| Cys-c (mg/dL) | 0.51±0.04 | 1.45±0.05 | 0.65±0.04 | 0.0001 | 0.001 | 0.001 | 0.0001 |
G+S=Gentamicin+saline; G+V=Gentamicin+vinpocetine; A=Control vs G+S; B=Control vs G+V; C=G+V vs G+S, MDA=Malondialdehyde; SOD=Superoxide dismutase; KIM-1=Kidney injury molecule-1; Cys-C=Cystatin; NGAL=Neutrophil gelatinase associated lipocalin
Effects of vinpocetine on the biochemical and inflammatory biomarkers in gentamicin-induced-AKI
Vinpocetine led to reduction of blood urea and serum creatinine compared with gentamicin group P < 0.01. Vinpocetine improved oxidative stress through reduction of MDA serum level and elevation of SOD significantly compared with gentamicin group P = 0.001 and P = 0.03, respectively. Indeed, vinpocetine reduced glomerular and renal tubular injury. Inflammatory biomarkers including KIM-1, NGAL, and Cystatin-c sera levels were reduced significantly P < 0.01 compared with gentamicin group [Table 1]. Moreover, co-administration of vinpocetine with gentamicin reduced gentamicin-induced-AKI inflammatory biomarkers as most of these biomarkers were not significantly differed compared to the control group except serum creatinine and cystatin-c serum levels which were not improved [Table 1].
Effects of vinpocetine on the estimated GFR on gentamicin-induced-AKI
In gentamicin-induced-AKI creatinine based-estimated GFR was significantly reduced to 51 ± 6.90 ml/min/1.73 m2 compared with 156.55 ± 10.45 ml/min/1.73 m2 in control group P = 0.0001 while; vinpocetine significantly increased it to 88 ± 7.11 ml/min/1.73 m2 compared with gentamicin group P = 0.001. As well, Cystatin based-estimated GFR was significantly reduced in gentamicin group to 60 ± 7.89 ml/min/1.73 m2 compared with the control group 166 ± 11.78 ml/min/1.73 m2 but; vinpocetine improve it up to 147 ± 9.78 ml/min/1.73 m2 compared with gentamicin group P = 0.001 [Figure 3].
Figure 3.
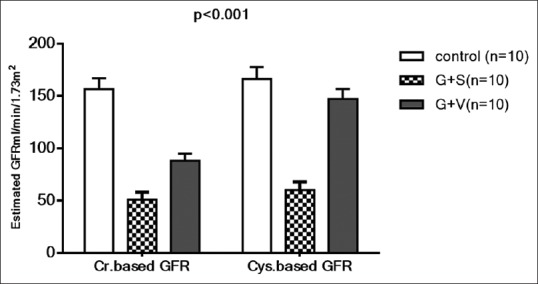
Effects of vinpocetine on creatinine based-estimated GFR and Cystatin based-estimated GFR in gentamicin-induced-AKI
Serum creatinine was not significantly correlated with biochemical and inflammatory biomarkers in gentamicin-induced-AKI in the control group P > 0.05. In gentamicin and vinpocetine groups serum creatinine positively and significantly correlated with blood urea, MDA, NGAL, and KIM-1 sera levels P < 0.01 but it negatively correlated with SOD serum levels, creatinine based-estimated GFR, and Cystatin based-estimated GFR P < 0.01 [Table 2].
Table 2.
Correlations of serum creatinine with biochemical and inflammatory biomarkers in gentamicin-induced-AKI
| Variables | Control (n=10) | Gentamicin (n=10) | Vinpocetine (n=10) | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Blood urea (mg/dL) | 0.62 | 0.05 | 0.92 | 0.0001* | 0.88 | 0.0007* |
| MDA (ng/mL) | 0.45 | 0.19 | 0.97 | 0.00001* | 0.87 | 0.001* |
| SOD (pg/mL) | 0.33 | 0.35 | −0.89 | 0.0005* | −0.88 | 0.0007* |
| KIM-1(pg/mL) | 0.36 | 0.30 | 0.97 | 0.00001* | 0.91 | 0.00025* |
| NGAL (pg/mL) | 0.47 | 0.17 | 0.95 | 0.00001* | 0.91 | 0.00025* |
| Cys-c (mg/dL) | 0.36 | 0.30 | 0.94 | 0.0001* | 0.82 | 0.003* |
| Cys-c GFR (ml/min/1.73) | 0.31 | 0.38 | −0.96 | 0.00001* | −0.91 | 0.00025* |
| Cr. GFR (ml/min/1.73) | 0.21 | 0.56 | −0.97 | 0.00001* | −0.84 | 0.002* |
*P<0.01; E-GFR=Estimated glomerular filtration rate; MDA=Malondialdehyde; SOD=Superoxide dismutase; KIM-1=Kidney injury molecule-1; Cys-C=Cystatin; NGAL=Neutrophil gelatinase associated lipocalin
Discussion
The present study illustrated a significant nephrotoxic effect of gentamicin through elevation of blood urea and serum creatinine as well as increment in the pro-inflammatory and oxidative stress biomarkers as indicated by a recent Al-Suleimani et al. study that confirmed gentamicin-induced-AKI.[15]
Additionally, gentamicin reduces renal blood flow which may contribute to renal proximal tubular damage. Gentamicin-induced-renal ischemia leads to induction of inducible nitric oxide synthase that causes mitochondrial oxidative stress and inhibition of ATP production in glomerular endothelial cells.[16] This may explain the induction oxidative stress and elevation of inflammatory biomarkers as well as significant reduction of estimated GFR of the present study.
Several studies indicate that the production of free radicals and induction of oxidative stress is the main important pathway of gentamicin-induced-AKI. Overproduction of reactive oxygen species is linked with reduction of anti-oxidant potential of proximal renal tubules which subsequently develop into lipid peroxidation and tubular damages.[17,18] These findings correspond with results of our study as gentamicin-induced-AKI was linked with high MDA and low SOD sera levels with significant elevation in KIM-1, NGAL, and Cyst-c sera levels which indicate acute oxidative stress in renal tubular injury and glomerular dysfunction.
Co-administration of vinpocetine with gentamicin in the present study significantly improved renal function as reflected by the reduction of blood urea and serum creatinine with significant amelioration of estimated GFR. This result indicates a renoprotective effect of vinpocetine. This is also documented by recent Fattori et al., study showing that vinpocetine improves diclofenac-induced AKI through modulation of NF-kB pathway, apoptosis, oxidative stress and cytokine productions.[19]
Vinpocetine in the present study illustrated potential anti-oxidant and anti-inflammatory effects on gentamicin-induced-AKI through reduction of MDA, NGAL, Cystatin-c and KIM-1 sera levels as demonstrated by different studies.[20,21]
Similarly, vinpocetine improved GFR which was due to inhibition of gentamicin-induced-oxidative stress since Ratliff et al., and Sverrison et al., studies confirmed the close relation between oxidative stress and deterioration of GFR.[22,23]
It has been documented that vinpocetine is a selective inhibitor of PDE-1 regulating cAMP and cGMP levels.[24] This selective inhibition does not participate in the renoprotective effective of vinpocetine as Thieme et al., study reported that vinpocetine did not increased PDE-1in renal vasculature and not increased renal blood flow following Ang II induced-renal dysfunction since; PDE-5 but not PDE-1 is predominant in renal vasculature.[25]
Additionally, previous study illustrated that gentamicin-induced-AKI was mediated through activation of rennin-angiotensin system mainly through up-regulation of Ang II which causes renal injury directly or through up-regulation of PDE-1 that lead to glomerular and endothelial dysfunctions.[26]
Overall, PDE inhibitor like vinpocetine improves renal and cardiac function via attenuation the effect of Ang II.[27] Moreover, Ang II inhibitors play potential role in attenuation of AKI in different model studies.[28] These findings are corresponding with results of the present study and give a clue about the renoprotective effect of vinpocetine which was PDE-dependent and independent mechanisms.
Conclusions
Vinpocetine leads to significant attenuation of gentamicin-induced-AKI through modulation of oxidative stress and pro-inflammatory pathway.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- 1.Vysakh A, Abhilash S, Kuriakose J, Midhun SJ, Jyothis M, Latha MS. Protective effect of Rotula aquatica Lour against gentamicin induced oxidative stress and nephrotoxicity in Wistar rats. Biomed Pharmacother. 2018;106:1188–94. doi: 10.1016/j.biopha.2018.07.066. [DOI] [PubMed] [Google Scholar]
- 2.Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai J, Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem Pharmacol. 2014;90:331–7. doi: 10.1016/j.bcp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 4.McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol. 2017;32:2015–25. doi: 10.1007/s00467-016-3533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada T, Nagai J, Okada Y, Yumoto R, Takano M. Gadolinium modulates gentamicin uptake via an endocytosis-independent pathway in HK-2 human renal proximal tubular cell line. Eur J Pharmacol. 2012;684:146–53. doi: 10.1016/j.ejphar.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Al-Kuraishy HM, Al-Gareeb AI, Al-nami MS. Pomegranate attenuates acute gentamicin-induced nephrotoxicity in sprague-dawley rats: The potential antioxidant and anti-inflammatory effects. Asian J Pharm Clin Res. 2019;12:1–3. [Google Scholar]
- 7.Bustos PS, Deza-Ponzio R, Páez PL, Albesa I, Cabrera JL, Virgolini MB, et al. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ Toxicol Pharmacol. 2016;48:253–64. doi: 10.1016/j.etap.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Wong HS, Chen JH, Leong PK, Leung HY, Chan WM, Ko KM. β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules. 2014;19:17649–62. doi: 10.3390/molecules191117649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagoly E, Fehér G, Szapáry L. The role of vinpocetine in the treatment of cerebrovascular diseases based on human studies. Orvosi Hetilap. 2007;148:1353–8. doi: 10.1556/OH.2007.28115. [DOI] [PubMed] [Google Scholar]
- 10.Alkuraishy HM, Al-Gareeb AI, Albuhadilly AK. Vinpocetine and pyritinol: A new model for blood rheological modulation in cerebrovascular disorders—A randomized controlled clinical study. BioMed Res Int. 2014;2014:324307. doi: 10.1155/2014/324307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: A review of the literature. Molecules. 2014;20:335–47. doi: 10.3390/molecules20010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega A, Rámila D, Izquierdo A, González L, Barat A, Gazapo R, et al. Role of the renin-angiotensin system on the parathyroid hormone–related protein overexpression induced by nephrotoxic acute renal failure in the rat. J Am Soc Nephrol. 2005;16:939–49. doi: 10.1681/ASN.2004040328. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–81. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 15.Al Suleimani YM, Abdelrahman AM, Karaca T, Manoj P, Ashique M, Nemmar A, et al. The effect of the dipeptidyl peptidase-4 inhibitor sitagliptin on gentamicin nephrotoxicity in mice. Biomed Pharmacother. 2018;97:1102–8. doi: 10.1016/j.biopha.2017.10.107. [DOI] [PubMed] [Google Scholar]
- 16.Ali BH, Al Za'abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: A mini-review of recent research. Basic Clin Pharmacol Toxicol. 2011;109:225–32. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 17.Alkuraishy HM, Al-Gareeb AI, Al-Naimi MS. Pomegranate protects renal proximal tubules during gentamicin induced-nephrotoxicity in rats. Journal of Contemporary Medical Sciences. 2019;5:35–40. [Google Scholar]
- 18.Al-Kuraishy HM, Al-Gareeb AI, Rasheed HA. Antioxidant and anti-inflammatory effects of curcumin contribute into attenuation of acute gentamicin-induced nephrotoxicity in rats. Asian J Pharm Clin Res. 2019;12:466–46. [Google Scholar]
- 19.Fattori V, Borghi SM, Guazelli CF, Giroldo AC, Crespigio J, Bussmann AJ, et al. Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-κB activation in mice. Pharmacol Res. 2017;120:10–22. doi: 10.1016/j.phrs.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: A review of the literature. Molecules. 2014;20:335–47. doi: 10.3390/molecules20010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvath B, Marton Z, Halmosi R, Alexy T, Szapary L, Vekasi J, et al. In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin Neuropharmacol. 2002;25:37–42. doi: 10.1097/00002826-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–46. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sverrisson K, Axelsson J, Rippe A, Asgeirsson D, Rippe B. Acute reactive oxygen species (ROS)-dependent effects of IL-1β, TNF-α, and IL-6 on the glomerular filtration barrier (GFB) in vivo. Am J Phys-Renal Physiol. 2015;309:F800–6. doi: 10.1152/ajprenal.00111.2015. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang J, Peng W, Li H, Lu Y, Wang K, Fan F, et al. Inhibitory effects of vinpocetine on the progression of atherosclerosis are mediated by Akt/NF-κB dependent mechanisms in apoE-/-mice. PLoS One. 2013;8:e82509. doi: 10.1371/journal.pone.0082509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thieme M, Sivritas SH, Mergia E, Potthoff SA, Yang G, Hering L, et al. Phosphodiesterase 5 inhibition ameliorates angiotensin II-dependent hypertension and renal vascular dysfunction. Am J Physiol-Renal Physiol. 2017;312:F474–81. doi: 10.1152/ajprenal.00376.2016. [DOI] [PubMed] [Google Scholar]
- 26.Funakoshi Y, Ichiki T, Takeda K, Tokuno T, Iino N, Takeshita A. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 2002;277:18710–7. doi: 10.1074/jbc.M110430200. [DOI] [PubMed] [Google Scholar]
- 27.Wu MP, Zhang YS, Xu X, Zhou Q, Li JD, Yan C. Vinpocetine attenuates pathological cardiac remodeling by inhibiting cardiac hypertrophy and fibrosis. Cardiovasc Drugs Ther. 2017;31:157–66. doi: 10.1007/s10557-017-6719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeisi S, Ghorbanihaghjo A, Argani H, Dastmalchi S, Ghasemi B, Ghazizadeh T, et al. Effects of angiotensin II receptor blockade on soluble klotho and oxidative stress in calcineurin inhibitor nephrotoxicity in rats. Iran J Kidney Dis. 2016;10:358–63. [PubMed] [Google Scholar]


