Figure 1.
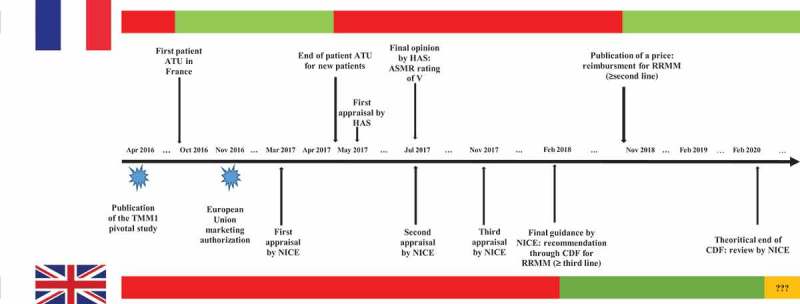
Comparative availability over time of ixazomib for new patients with relapsed or refractory multiple myeloma between France and England and main milestones of the appraisal. The red bars denote periods of time during which ixazomib was not reimbursed in each country's health-care system, the green bars denote periods of time during which ixazomib was reimbursed in each country’s health-care system, the orange bar denotes potential uncertainty in the reimbursement of ixazomib.TMM-1 = Tourmaline MM1 trial; NICE = National Institute for Health and Care Excellence; HAS = French High Health Authority; CDF = Cancer Drugs Fund; ASMR = improvement of actual medical benefit; RRMM = relapsed refractory multiple Myeloma; ATU = Early Access Program.
