Fig. 2. Senescence and SASP induction after combination MEK and CDK4/6 inhibitor therapy induces NK cell immune surveillance.
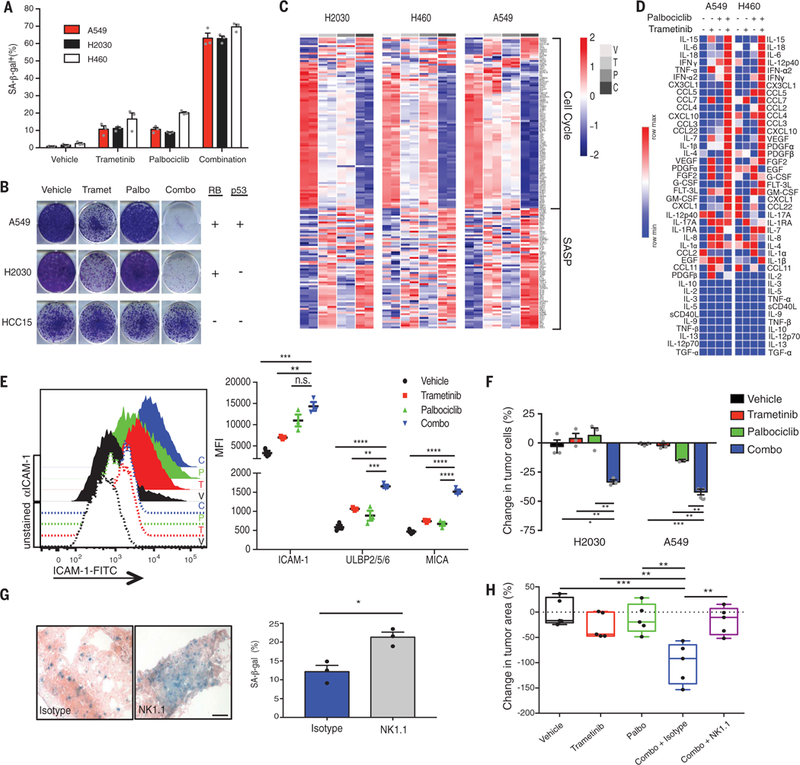
(A) Quantification of SA-β-gal+ cells in human KRAS-mutant lung tumor cell lines after 8-day treatment with trametinib (25 nM) and/or palbociclib (500 nM). Mean of three biological replicates is plotted. (B) Clonogenic assay of human KRAS-mutant lung cancer cells replated in the absence of drugs after 8-day pretreatment as in (A); representative of three biological replicates. RB and p53 genomic status is indicated on right. (C) Heat map of senescence-associated cell cycle and SASP gene expression in human KRAS-mutant lung cancer cell lines after treatment as in (A), as assessed by RNA-seq.Two biological replicates per cell line are shown. C, combination; P, palbociclib; T, trametinib; V, vehicle. (D) Heat map of cytokine array results from human KRAS-mutant lung tumor cells treated with trametinib (25 nM) and/or palbociclib (500 nM) for 8 days. Data are presented as mean of three biological replicates. (E) Flow cytometry analysis of ICAM-1 expression and levels of other NK cell–activating ligands after 8-day treatment of A549 lung tumor cells as described in (A). Quantification of mean fluorescence intensity (MFI) from three biological replicates is shown on the right. (F) Quantification of NK cell cytotoxicity (by live cell imaging) after pretreatment of A549 and H2030 lung tumor cell lines with indicated drugs for 8 days and coculturing with the YT NK cell line at a 10:1 effector to target cell (E:T) ratio for 20 hours in the presence or absence of indicated drugs. Change in tumor cells is normalized to control wells lacking NK cells. Mean of three biological replicates is plotted. (G) Representative SA-β-gal staining of KP transplant lung tumors after 1-week treatment with combined trametinib (1 mg/kg body weight) and palbociclib (100 mg/kg body weight) and either an isotype control antibody (C1.18.4) or NK1.1 depleting antibody (PK136) (scale bar, 50 mm). Quantification shown on right (n = 3 mice per group). (H) Effect of 1-week treatment as in (G) on lung tumor burden (relative to vehicle) (n = 5 mice per group). (E, F, and H) One-way ANOVA. (G) Unpaired two-tailed t test. Error bars, mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
