Fig. 4. Combination trametinib and palbociclib treatment drives NK cell–mediated lung tumor regressions in genetically engineered mice.
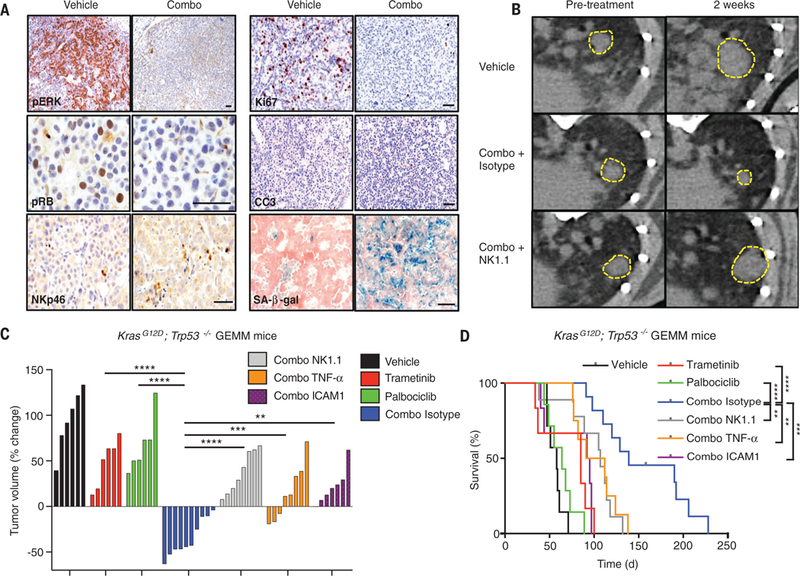
(A) Immunohistochemical staining of KP GEMM tumors treated with vehicle or combined trametinib (1 mg/kg body weight) and palbociclib (100 mg/kg body weight) for 2 weeks (scale bar, 50 μm). CC3, cleaved caspase-3; pERK, phosphorylated extracellular signal–regulated kinase. (B) Representative μCT images of KP GEMM lung tumors prior to treatment and after 2 weeks of treatment with vehicle or combined trametinib (1 mg/kg body weight) and palbociclib (100 mg/kg body weight) and either an isotype control antibody (C1.18.4) or NK1.1 depleting antibody (PK136). Yellow boxes indicate lung tumors. (C) A waterfall representation of the response of each tumor after 2 weeks of treatment with vehicle, trametinib (1 mg/kg body weight), palbociclib (100 mg/kg body weight), or both, and either an isotype control (C1.18.4), NK1.1 (PK136), TNF-α (XT3.11), or ICAM-1 (YN1/1.7.4) blocking antibody (n ≥ 6 per group). (D) Kaplan-Meier survival curve of KP GEMM mice treated as in (C) (n ≥ 6 per group) (log-rank test). (C) One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
