Abstract
Ebolavirus (EBOV) has caused disease outbreaks taking thousands of lives, costing billions of dollars in control efforts and threatening great ape populations. EBOV ecology is not fully understood but infected wildlife and consumption of animal carcasses have been linked to human outbreaks, especially in the Congo Basin. Partnering with the Congolese Ministry of Health, we conducted wildlife mortality surveillance and educational outreach in the northern Republic of Congo (RoC). Designed for EBOV detection and to alert public health authorities, we established a low-cost wildlife mortality reporting network covering 50 000 km2. Simultaneously, we delivered educational outreach promoting behavioural change to over 6600 people in rural northern RoC. We achieved specimen collection by training project staff on a safe sampling protocol and equipping geographically distributed bases with sampling kits. We established in-country diagnostics for EBOV testing, reducing diagnostic turnaround time to 3 days and demonstrated the absence of EBOV in 58 carcasses. Central Africa remains a high-risk EBOV region, but RoC, home to the largest remaining populations of great apes, has not had an epidemic since 2005. This effort continues to function as an untested early warning system in RoC, where people and great apes have died from past Ebola virus disease outbreaks.
This article is part of the theme issue ‘Dynamic and integrative approaches to understanding pathogen spillover’.
Keywords: surveillance, carcass, Ebola spillover, One Health, community outreach, great ape
1. Introduction
Ebolavirus (Zaire ebolavirus; EBOV) has caused high-mortality outbreaks in both humans and wildlife. First identified in 1976 in the Democratic Republic of Congo (DRC), recurrent epidemics have impacted central and western African regions. The 2014–2016 West African epidemic resulted in over 11 000 human deaths and was declared a Public Health Emergency of International Concern, requiring extensive international response [1]. In the central African region, smaller scale outbreaks have occurred frequently over the last decades in Gabon, the Republic of Congo (RoC) and the DRC [2]. Since 2017, three outbreaks of Ebola virus disease (EVD) have occurred in the provinces of Bas Uele, Equateur and North Kivu of the DRC [3]. The associated control efforts have amassed huge financial and social costs for the affected countries and the international public health sector. The cost of controlling the 2018 epidemic in Equateur, DRC, alone mounted to an estimated US$100 million. Aside from the devastating health effects, the EVD epidemics have pronounced socio-economic impacts.
EBOV has had a similarly devastating impact on African great ape populations. Concurrent with the human EVD epidemics in the mid-1990s and early 2000s, biologists reported mass die-offs of western lowland gorillas (Gorilla gorilla gorilla) and chimpanzees (Pan troglodytes) in RoC, Cameroon and Gabon, and EBOV was detected in a number of these carcasses [4–6]. Estimating the true effect of EBOV on the region's great ape populations is difficult owing to limited ability to detect carcasses in an expansive forested landscape and the challenges of accurate estimation of the size of wild great ape populations, but the impact of EBOV on a local scale was devastating. In the 40 km radius of the Lossi reserve in the RoC, EVD was estimated to have killed 5000 great apes, and surveys of several closely monitored gorilla populations concluded that in some areas local populations experienced mortality reaching 91–96% [5,7]. After the EVD outbreaks in the RoC and Gabon border areas, between 2005 and 2008, anti-EBOV antibodies were detected in 10% of great ape fecal samples collected in the nearby Odzala-Koukoua area in the RoC [8]. In the light of these events, the International Union for Conservation of Nature subsequently classified the western lowland gorilla as critically endangered and chimpanzee as endangered, largely owing to the catastrophic potential of infectious diseases, EBOV in particular, on already threatened populations [9]. Addressing infectious disease threats has since become an indispensable part of the agenda for great ape conservation in the central African region, which hosts the majority of the world's remaining great apes [10].
EBOV spills over into the human population through direct contact with infected wild animals. Investigations into the Gabon-RoC epidemics between 2001 and 2005 revealed that five separate human outbreaks were initiated from isolated introductions of the virus from different infected animal sources [6,11,12]. Such epidemiological connections between hunters and likely infected wildlife carcasses were demonstrated in most spillover events of EBOV that gave rise to human EVD outbreaks [13]. The index cases in several human outbreaks have been hunters, butchers or people frequently handling raw meat; many with a confirmed or suspected preceding contact with an infected wildlife carcass. Gorillas, chimpanzees, monkeys, duikers, wild hogs and bats all have suspected epidemiological links to human outbreaks [11,13,14]. Consumption of carcass meat for food is a common practice and the hunter communities are inherently at a risk of EBOV exposure. Environmental and socio-economic factors further exacerbate the zoonotic spillover risk [2]. Outbreaks of emerging zoonotic diseases are also increasingly common [15]. Extractive industries and expanding human encroachment into wild areas increase the close interactions between human and wildlife populations [16]. Ensuing ecosystem impacts can affect disease ecology, simultaneously heightening the risk of disease spillover from natural host species to other wildlife or to human populations [17].
For these reasons, early detection of a zoonotic threat, such as an EBOV spillover event, is an important consideration for public health [18]. The expanding spatial connectivity and mobility of the central African population mean that epidemics, like EVD, can spread rapidly [2]. Combined with increasingly large urban populations, such events can overwhelm the public health infrastructure and response efforts. Early detection can limit such catastrophic spread and as wildlife epizootics often precede human epidemics, disease surveillance in wildlife presents an inherent advantage. It may enable detection of zoonotic pathogens before the pathogens have an opportunity spillover into human populations and thus the deployment of control measures [19,20]. However, environmental conditions can present a notable challenge to wildlife surveillance efforts. In central Africa's extensive and dense forest, active surveillance of wildlife mortalities is difficult and expensive. During previous EVD epizootics, passive surveillance by hunters and field biologists generated the majority of carcass reports, leading to detection of EBOV in wildlife before human outbreaks [5,6]. Carcass sampling has yielded the highest prevalence of virus detection, over 150 times more than live capture [21].
Coupling surveillance with community education and outreach that addresses the individuals most likely to encounter risk at the human–wildlife interface can effectively impact both the spillover and epidemic spread [22–24]. In the Congo basin where a large proportion of the rural population relies on bushmeat hunting as a primary source of protein, a culturally sensitive educational campaign can encourage a behavioural change that may in turn reduce the risk of zoonotic disease spillover. Targeted education campaigns aimed at the high-risk population groups can similarly reduce the incidence and the consequences of an outbreak.
Here, we describe the design and implementation of a community-based wildlife mortality surveillance network originally designed for early detection of EVD epizootics, and combined with an educational community outreach programme. We partnered with the RoC Ministry of Health to implement this low-cost and wide-coverage network, and explain the role this community-based surveillance system has played as an early warning system for a pathogen affecting both human and wildlife health. We also examine whether reporting great ape and other mammal carcasses by local communities performed effectively as a wildlife mortality surveillance network and could assist with a quickened public health response. This programme has particular relevance in northern RoC, where a number of endangered wildlife species are still found in significant numbers and where outbreaks of EVD have occurred. Fortunately, RoC has not detected a human epidemic of EVD since 2005 [12]. However, neighbouring DRC has endured seven outbreaks in this time, demonstrating that this region continues to be an endemic, high spillover-risk zone for EBOV.
2. Framework for the wildlife mortality surveillance
The framework of our wildlife disease surveillance in the RoC comprises three core elements. These include: the establishment and maintenance of a wide-coverage wildlife mortality reporting network; the building and maintenance of capacity for safe carcass sampling across that network and rapid response to wildlife mortality reports, including fast diagnostics for the detection of EBOV and reporting of results back to local communities. Educational outreach was incorporated to reduce the risk of a zoonotic spillover event and spread and to explain the purpose of the wildlife mortality surveillance network to its rural community partners. Coordination and a clear communication strategy across the entire surveillance operation were essential. Below, we explain the rationale and methodology of each element and describe perceived outcomes of the programme.
3. Establishment and maintenance of a wide-coverage wildlife mortality reporting network
Wildlife mortalities are easily unnoticed in tropical forests and environmental conditions facilitate rapid decomposition of carcasses. Many previous EVD spillover events occurred in sparsely populated areas with low levels of human activity, yet these are the high-risk areas of key interest for surveillance. Active surveillance conducted for the purpose of locating carcasses is time-consuming and prohibitively expensive for large areas. Meanwhile, the main sources of carcass reports during previous epizootics were local hunters and researchers [5]. By encouraging cooperation with local communities and existing ‘boots on the ground’ that regularly cover a wide geographical area, passive surveillance has the potential to be very cost-effective.
We partnered with the RoC Ministry of Health to launch a community outreach campaign designed to build a wildlife mortality reporting network across northern RoC. By engaging with hunter–gatherers in forests to collectively form a wildlife mortality reporting network, we also addressed a high-risk interface for zoonotic spillovers. Combining an educational campaign to promote behavioural change in these populations directly targeted the risk behaviours leading to spillover events.
The outreach campaigns were run on a mission-based structure, with each multi-day mission consisting of visits to several villages in pre-defined target regions. We identified and trained local professionals and community members for the outreach teams to ensure our methods and message were culturally appropriate, and to foster positive and long-term relationships with communities. We initially prioritized villages in the areas of the most recent human EVD outbreaks (figure 1) and the number of villages visited during each mission depended on geographical and logistical limitations. At each visit, our outreach team first approached the village chief for permission to address the village community, a prerequisite to effectively engage communities in the region. The outreach team then delivered the educational agenda to all interested and available community members, including men, women and children. An important component of the programme's educational message was to dispel the widely held belief that EVD was the result of sorcery, and the message, delivered verbally, included a presentation on the characteristics, ecology and history of EVD, and the potential threat it poses to the wildlife and the human population. We paid particular attention to explaining the risk associated with close contact or consumption of meat originating from carcasses. We guided the villagers on how to act when finding a carcass and how to avoid possible exposure. We emphasized the core message in a deliberately simple and clear manner: do not touch, move or bury the carcass and contact the surveillance network immediately.
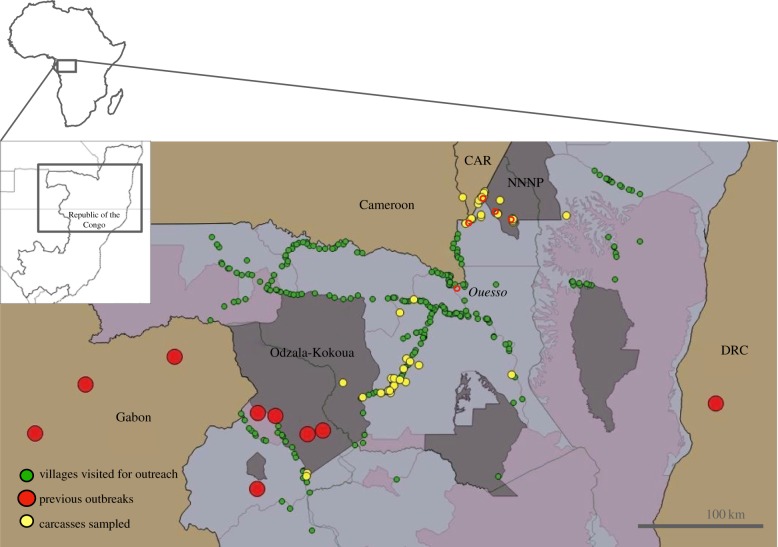
Figure 1.
Community-based carcass surveillance network in the RoC. Confirmed EBOV outbreaks in the northern DRC-RoC-Gabon-Cameroon region (large red dots), the villages visited for educational outreach between April 2008–September 2018 (green, n = 268) and the GPS locations of carcasses sampled and analysed for the presence of EBOV (yellow). Protected areas are highlighted in grey. CAR, Central Africa Republic; NNNP, Nouabalé-Ndoki National Park. The sampling station (red circles) distribution reflects partly the accessibility of the terrain. Ouesso base (named, with red circle) can reach the villages south of the base within 1–2 days. Longest distances that our teams have travelled to sample a carcass have been over 200 km from the Ouesso base. In the national parks, carcasses only 30 km away may take over a day to reach. In these areas, the sampling bases have been set up more frequently into the available camps.
Our outreach team instructed the community members and village chief on how to effectively report wildlife mortalities, and explained the use of a carcass reporting forms designed to record information on the carcass finding. However, the use of the forms was never a prerequisite for reporting a carcass. We provided each village with a set of educational posters (electronic supplementary material, appendix S1), printed both in French and Lingala (a native language of northern RoC), and these were placed in visible locations in communal buildings. During the meetings, we gave the villagers an opportunity to share their experiences and ask questions relating to the topic and these discussions were used to reinforce the educational message. To further amplify the outreach message and re-engage the contacted villagers in our carcass surveillance programme, we broadcast a local radio campaign reinforcing the key messages and information on how to report carcasses to the network.
4. Building capacity for rapid and safe carcass sampling across a wide geographical area
Several carcass sampling and diagnostic methods have been used since our veterinary team first responded to a 2004 great ape Ebola mortality event, including acquisition of necropsy samples by trained personnel, and analysis of the diagnostic samples at the Medical Research Institute of Franceville, Gabon (CIRMF). Here, we describe the sampling and diagnostic methodology implemented in January 2017. This latest iteration of the methods is based on the lessons learned over the course of the programme with effective and safe surveillance in mind, and is the basis of the current wildlife surveillance strategy for EBOV. Description of the previous sampling and diagnostic methodology can be found in the electronic supplementary material, appendix S2.
We trained 16 project staff based at different locations across northern RoC on a two-person protocol for minimal exposure sampling of wildlife carcasses (electronic supplementary material, appendix). This protocol, available in both English and French, combines methods deployed and proven in human EVD outbreak situations with the limitations imposed by a remote field environment. Key characteristics of the protocol are multiple layers of redundant biosafety and a buddy system whereby the samplers oversee each other's tasks, donning and doffing of the personal protective equipment (PPE), sampling and disinfection. Rather than requiring solid tissue specimens or the use of sharp objects and substantial equipment, this method relies on minimally invasive swab sampling and a de-activating buffer that minimizes the samplers', the downstream handlers' and transporters’ exposure risk and results in a high quality diagnostic sample [25].
The training included theoretical information on the history, ecology and dynamics of EBOV and the nature, epidemiology and control of EVD outbreaks in humans. Practical teaching consisted of repeated practice of the protocol and scenario-based training (figure 2). Refresher training courses were organized to available personnel and less experienced samplers were chosen to accompany the experienced samplers on sampling missions to gain experience.
Figure 2.
Clockwise from top: deployable complete carcass sampling kit with instructions; teams practice sampling during training; scavenged and decomposed remnants of a week-old juvenile gorilla carcass. (Online version in colour.)
Our project bases in Brazzaville, Ouesso and Bomassa, and research camps in the Nouabalé-Ndoki National Park (NNNP) (Mondika, Goualougo and Mbeli) were equipped with ready-to-deploy carcass sampling kits to aid rapid mobilization of the sampling team. The kits included pre-packaged single use PPE, sampling materials and the reusable equipment dedicated to carcass sampling (table 1 and figure 2). As long intervals can occur between carcass events, each kit included a complete guide with instructions to refresh samplers on the steps between the first report of a carcass event and transporting the sample after collection to facilitate fast diagnosis.
Table 1.
Complete carcass sampling kit for two-person minimally invasive carcass sampling protocol. (Several sets of the disposable PPE and sample collection materials were given to each camp to enable sampling multiple carcasses in the same time period. Replacement kits were sent to camps from a central stock at the project headquarters to maintain sampling readiness at each base.)
| disposable PPE | |||
|---|---|---|---|
| 2 | Tyvek™ hooded coverall | 2 | N95 respirator mask |
| 8 | pairs of nitrile gloves | 2 | pair of boot covers |
| sample collection materials | |||
| 4 | 2 ml vials of 1 ml AVL | 4 | biohazard bags |
| 4 | polyester-tipped swabs | 1 | 3 m plastic line to establish sampler boundary |
| 3 | sample tube bags, labelled | 4 | zip ties |
| 1 | carcass sampling ID card | 4 | bleach tablets |
| 1 | plastic bag for sample card | 2 | pairs nitrile gloves |
| 1 | notebook page-format form for carcass details | 1 | N95 respirator |
| 1 | transport container label | ||
| reusable materials | |||
| 1 | 3–5 l pump sprayer | 1 | machete or secateur |
| 1 | 5 l plastic bucket | 1 | 10 l of water |
| 1 | roll of duct tape | 1 | GPS device |
| 1 | 10 l can, for water | 1 | pen or pencil |
| 2 | goggles | 1 | watch |
| 1 | plastic base | 1 | hand sanitizer/soap |
| 1 | sample transport container | 1 | insecticide sprayer (disposable) |
5. Rapid response to wildlife mortality events and fast diagnostics for the detection of Ebolavirus
On detection of a carcass, a village member or partnering organization contacted us, usually by telephone. In 2017, we created a dedicated carcass reporting hotline connected to a central office to facilitate clear and timely reporting. In the affiliated protected areas reporting was done via satellite or VHF radio communications to central control rooms within the park from where the information was then relayed via telephone to us. A report immediately initiated a carcass sampling mission.
Location details of the carcass such as reporting village or GPS coordinates (depending on the source of the report) were recorded. The trained carcass responder team confirmed further details upon sampling and photographing the carcass. Sampling visits were used to reinforce educational messages and the relationship between the village communities and the surveillance team. The costs of reporting the carcass (telephone call) and a compensation for the effort given to facilitate the sampling teams' mission were offered to the village communities, and they were again reminded about the potential risks relating to the carcass, and the need to report any additional incidences in the area.
The sampling teams were generally comprised a minimum of five people (two local porters/trackers, a person who knows the exact location of the carcass and two trained samplers). The samples were collected following the two-person sampling protocol (electronic supplementary material, appendix S2). Depending on the sampling location and available materials, samples were transported from the collection site at ambient temperature or inside a polystyrene transport box with frozen gel packs to the testing laboratory in Brazzaville, RoC.
We achieved capacity for quantitative real-time polymerase chain reaction (qRT-PCR) analysis for the detection of EBOV at the National Public Health Laboratory (NPHL) in Brazzaville. The method used RNA extraction in a class III biosafety cabinet and qRT-PCR methods, as described previously [26] (electronic supplementary material, appendix S3). Furthermore, personnel at the NPHL were trained in biosafety and deployment of a fully functional mobile diagnostic laboratory for human EVD outbreaks, and we have recently established baseline capacity for sequencing the full EBOV genome using the Oxford Nanopore Technologies portable MinION nucleic acid sequencing platform [27].
All test results initiated a repeat visit or a telephone call with the reporting community to share the result and to reinforce the outreach message.
6. Results and discussion
Here we describe the operation of a community-based surveillance system, coupled with an educational outreach programme for EBOV in the northern RoC. The programme was designed to function as an early warning system to enable surveillance and detection of EBOV epizootics. An epizootic would warrant dissemination of information to the public health authorities and local communities for the mobilization of preventative and control measures to curb a possible human EBOV outbreak. This One Health aligned surveillance helps position both wildlife conservation and public health focused organizations to respond with mitigative actions to protect threatened human and great ape populations.
Between April 2008, when documentation on the visits began, and September 2018, we made a total of 520 visits to 268 villages over 26 separate missions in the RoC departments of Cuvette Ouest, Cuvette, Sangha and Likouala (figure 1). We delivered the educational outreach message to a total of 6658 hunters, and to thousands of women and children who frequently visit the forest for gathering. Many villages were re-visited annually, and some individuals may have attended a session more than once. The average number of people attending an outreach meeting was 12.8 (range 1–127, median was 10). Collectively, we estimate our community-based surveillance network covered 29 800 km2 of forested areas in these regions, based on an assumed non-overlapping 10 km radius of hunting and foraging activities from the home villages. In addition, our affiliated project personnel and teams of rangers in the NNNP patrolled an estimated 75 000 km of trails in the protected park areas and peripheries in 2017–2018 alone, covering a total area of 24 200 km2. Cooperating industry projects operating in the geographical area cover in excess of 16 000 km2 of forested logging and hunting concessions. In total, we estimate our surveillance area covers 50 200 km2.
Engagement with other organizations and private industries operating in the geographical area enabled expansion of the surveillance area. Cooperation on surveillance is of mutual interest as the partnering organizations are responsible for the well-being of their forest-based personnel and benefit from our educational programme and early warning system.
Anecdotally, we noticed carcass reports appeared more frequently in the areas where our presence was repeated and amplified, or where our teams have permanent activities. This highlights the importance of building a trusting relationship with the communities and collaborating organizations. Conversely, several communities did not report carcasses to us during this period. The threshold to reporting must be looked at in the context of the difficulties of doing so in the rural Congo, and reporting could be affected by factors such as educational status, level of economic dependence on bushmeat [28] or availability of a mobile network. However, if some carcasses were missed, we may have built critical baseline awareness for the rural communities to alert us in case of a larger die-off. Message reinforcement and a clear, consistent reporting system are important to facilitate continued reporting.
Effective surveillance requires rapid deployment of sampling teams to the carcass site once it is detected. Responding to carcass reports over a wide surveillance network area with limited infrastructure and resources poses a challenge, especially when technically skilled personnel capable of conducting the sampling are often in short supply. Fast decomposition of carcasses often presented a challenge in remote areas with demanding terrain, poor roads or no roads! While viral RNA is shown to be readily detectable in swab samples taken from carcasses for 21 days post mortem [25], even large mammal carcasses can be reduced to only dry skeletal remains within this time owing to a combination of scavenging and consumption of soft tissues by maggots, narrowing the window for successful recovery of diagnostic specimens.
We identified the use of existing project personnel across the surveillance area as the optimal way to achieve a reliable carcass response capacity. However, successful implementation required overcoming technical constraints and critical safety considerations. To address this, we developed a detailed sampling protocol designed for safe collection of diagnostic samples from EBOV suspect carcasses. Rather than requiring the presence of veterinary personnel at the carcass site, the protocol could be used by trained personnel without specialist biosafety background or experience. We trained 16 carcass responders stationed in different sites in the northern Congo to this protocol.
The training was successful in providing an accessible method for the samplers even when intervals between samplings were long. We also facilitated the rapid collection of the diagnostic samples by equipping strategic locations with readily deployable sampling kits and clear instructions. The locally responding teams enabled us to sample carcasses more reliably and days earlier than would have otherwise been possible in difficult-to-reach locations. Spatial distribution of carcass sampling capacity not only saved time, but also reduced logistical costs.
The network alerted us to multiple carcasses, and we demonstrated the functioning report-sampling-response-analysis process. Hunter communities aware of our veterinary team's presence in the area first reported carcasses to us in 2006, before the documented outreach programme began 2 years later in 2008. In total, between November 2006 and March 2018 we responded to 58 carcass reports (electronic supplementary material, appendix S5). Of these 21 were reported by village community members; 10 by the rangers patrolling the protected areas and hunting zone peripheries of NNNP and Odzala-Koukoua National Park; and 26 by the research and surveillance staff during missions in the protected areas and peripheries. We sampled carcasses from gorillas (n = 41), chimpanzees (n = 10), duikers (n = 2) and other mammal species (n = 4). The sampling teams mobilized and sampled the carcasses within 1–2 days of receiving the report. Six different trained individuals that were deployed from four different bases sampled the carcasses.
The samples were transported to the central testing laboratory in Brazzaville with minimum delay. The samples were transported refrigerated wherever cold chain was available. While this was not a prerequisite for the immediate transport, it prolongs the stability of viral nucleic acid [29]. At the beginning of the surveillance programme, exporting samples for testing stretched the diagnostic turnaround time to several weeks. Establishment of the diagnostic capacity within the country enabled us to reduce this time, however, periodic unavailability of skilled staff for the analysis prolonged the turnaround time, on two occasions for over four weeks. While there was variation, we could achieve diagnostic turnaround times of three days, and times from receiving a report of a carcass to producing a qRT-PCR result to four days. To date, all 58 samples collected from the carcasses were tested negative for EBOV.
A molecular testing laboratory capable of confirming the presence or the absence of EBOV in a carcass sample is a prerequisite for an effective disease surveillance system. Further, fast diagnostic turnaround time is essential to alert local and government public health officials and initiate an effective early response. Availability of diagnostic personnel capable of analysing the sample delayed some results. Directly addressing the causes of delays will improve the efficiency of the early warning system, such as training a wider pool of diagnostic personnel and diversifying the diagnostic locations to reduce transport time. Strengthening the in-country diagnostic capacity is fundamental for establishing a reliable and efficient early warning surveillance system. Additionally, development of rapid, portable carcass-side diagnostic tools could provide a method for fast detection of EBOV, and subsequently assist in decision-making regarding carcass disposal and further invasive necropsy procedures.
The absence of human outbreaks in RoC since our surveillance was implemented is in agreement with our EBOV-negative carcass findings. That said, the efficacy of this early warning system remains difficult to quantify—RoC has not had a confirmed EBOV epidemic since 2005. Designed as a surveillance effort we did not establish control communities to compare with the communities included in the network system that received educational exposure. Presently, we have no method to determine if community education efforts resulted in changed behaviour or avoided cases of EBOV spillover to humans. However, evaluating our outcomes to date helps highlight strengths, weaknesses and further opportunities.
The educational agenda was incorporated into the programme to explain the purpose of the surveillance and to provide reliable information on preventing spillover within the high-risk population. Communities received behavioural tools that enabled individuals to reduce their own risk of infection, but the extent of behavioural change has not been systematically studied. While difficult to demonstrate, the education and outreach work in northern Congo may have succeeded in delivering a degree of behavioural change in the hunter communities that in turn may have prevented zoonotic spillovers from occurring between 2005 and the present. In the future, an accurate assessment could involve a systematic sociological survey alongside outreach and pre- and post-assessments of the outreach itself.
Contrasted with the high costs of EVD outbreak containment, early detection is probably a cost-effective tool to strengthen outbreak preparedness efforts and mitigate human outbreaks. Enumerating the cost of this community-based surveillance was rife with issues that were debated among the co-authors. The surveillance was built on existing core conservation programming with strong veterinary expertise, infrastructure, capacity and well-trained field personnel distributed across the surveillance area. The time and investment needed to develop the institutional capacity and trust necessary to build and operate a national conservation programme effectively in central Africa cannot be overlooked. Our country programme's annual budget in 2017–2018 was approximately $US 11 million. These sums aside, we estimate the initial implementation of the village visits and basic level of engagement with the rural communities cost on the order of $US 30 000. Thereafter the maintenance, amplification and strengthening of the message is further economical at an estimated $US 3000 per annum. The radio campaigns can reach wide audiences with one-time associated costs and cooperation with organizations already on the ground is a valuable tool for improving cost-effectiveness. These estimates exclude existing core conservation-focused programming costs and we provide them only to provoke discussion about the potential value of surveillance efforts, as well as to recognize a co-benefit of past and current conservation investments in central Africa.
Finally, such wildlife mortality surveillance systems can serve a dual purpose. Acquisition of comprehensive health information for free ranging great apes and other mammals, and the implementation of disease investigations is challenging, partly owing to the remoteness of the forested regions. Parallel to the targeted EBOV surveillance, the reporting network can offer access to information on the causes of wildlife mortalities. With further investment into the diagnostic processes, next-generation sequencing analysis for the detection of bacterial and viral pathogens in the carcass samples could provide more answers. The mortality reporting network could be used to answer questions of veterinary health and conservation interest as well as pathogen discovery for yet unknown pathogens of One Health interest.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the Wildlife Conservation Society Congo Program for support; Dr David Morgan, Dr Crickette Sanz, Ivonne Kienast, Severin Ndassoba and Juan Ortega for assistance with sampling carcasses; Dr Terry Brncic and Aymard Ebah for geographical information and figures; and Sebastien Assoignons for photographs. Additional support has been provided by the RoC National Public Health Laboratories (NPHL) Department of Molecular Biology, the United States national Institutes of Health (NIH) and the Bernhard Nocht Institute for Tropical Medicine (BNITM) and German Society for International Cooperation (GIZ).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material. The figures were produced using QGIS 2.18 Las Palmas software (Free Software Foundation, Inc., 51 Franklin Street, Fifth Floor, Boston, MA, USA).
Authors' contributions
E.K. and S.H.O. drafted the initial manuscript; K.N.C., P.E.R., W.B.K., V.J.M., S.N.S. and J.V.M. helped revise the manuscript; E.K., S.H.O., K.N.C., P.E.R., A.I.O., R.J.F. and V.J.M. carried out development of the 2017 carcass sampling protocol and training; E.K., A.I.O., K.N.C., P.E.R., M.J.A. and S.D.K. helped establish or maintain the wildlife reporting network and the outreach programme; E.K. and S.H.O. participated in data analysis; E.K., S.H.O., K.N.C., P.E.R., A.I.O., M.J.A. and S.K. participated in the development of the surveillance programme; E.K., V.J.M., R.J.F., S.N.S., C.G.N., C.M.F., B.B.Z. and B.E.P. carried out the diagnostic analyses and training of staff for the diagnostics procedures and biosafety; all authors gave final approval for publication.
Competing interests
The authors declare no conflicts of interest related to this submission.
Funding
This work was supported by the United States Fish and Wildlife Service (Great Ape Conservation Fund), the Arcus Foundation, the Paul G. Allen Family Foundation, Mr and Mrs Bradley L. Goldberg, CSR Tullow Oil Society and the Réseau des Aires Protégées d'Afrique Centrale (RAPAC). The study also benefited from the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT project and German Research Foundation (DFG) grant no. MU 3565/3-0 and German Federal Ministry for Economic Cooperation and Development (BMZ) funding. B.J.F., S.N.S. and V.J.M. are supported by the Division of Intramural Research of the NIAID, NIH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript and the findings do not represent the opinions of the US government.
Disclaimer
Any opinions and conclusions are those of the author(s) and do not represent the official views of the U.S. Fish and Wildlife Service or USAID.
References
- 1.Spengler JR, et al. 2016. Perspectives on West Africa Ebola virus disease outbreak, 2013–2016. Emerg. Infect. Dis. 22, 2013–2016. ( 10.32032/eid2206.150021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, et al. 2018. Outbreaks in a rapidly changing central Africa: lessons from Ebola. New Engl. J. Med. 379, 1198–1201. ( 10.1056/NEJMp1807691) [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2017–2019 Situation Reports 2017–2019. See http://www.who.int/ebola/en/.
- 4.Georges AJ, et al. 1999. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues. J. Infect. Dis. 179, 65–75. ( 10.1086/514290) [DOI] [PubMed] [Google Scholar]
- 5.Bermejo M, Rodriguez-Teijeiro JD, Illera G, Barroso A, Vila C, Walsh PD. 2006. Ebola outbreak killed 5000 gorillas. Science 314, 1564 ( 10.1126/science.1133105) [DOI] [PubMed] [Google Scholar]
- 6.Rouquet P, et al. 2005. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 11, 283–290. ( 10.3201/eid1102.040533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caillaud D, et al. 2006. Gorilla susceptibility to Ebola virus: the cost of sociality. Curr. Biol. 16, 489–491. ( 10.1016/j.cub.2006.06.017) [DOI] [PubMed] [Google Scholar]
- 8.Reed PE, et al. 2014. A new approach for monitoring Ebolavirus in wild great apes. PLoS Negl. Trop. Dis. 8, 3143 ( 10.1371/journal.pntd.0003143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkin M. 2007. Gorillas on the list. Nature 449, 127 ( 10.1038/449127a) [DOI] [PubMed] [Google Scholar]
- 10.Strindberg S, et al. 2018. Guns, germs, and trees determine density and distribution of gorillas and chimpanzees in Western Equatorial Africa. Sci. Adv. 4, eaar2964 ( 10.1126/sciadv.aar2964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroy EM, et al. 2004. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 16, 387–390. ( 10.1126/science.1092528) [DOI] [PubMed] [Google Scholar]
- 12.Nkoghe D, Kone ML, Yada A, Leroy E. 2011. A limited outbreak of Ebola haemorrhagic fever in Etoumbi, Republic of Congo, 2005. Trans. R. Soc. Trop. Med. Hyg. 105, 466–472. ( 10.1016/j.trstmh.2011.04.011) [DOI] [PubMed] [Google Scholar]
- 13.Judson SD, Fischer R, Judson A, Munster VJ. 2016. Ecological contexts of index cases and spillover events of different Ebolaviruses. PLoS Pathog. 12, e1005780 ( 10.1371/journal.ppat.1005780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leroy EM, et al. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576. ( 10.1038/438575a) [DOI] [PubMed] [Google Scholar]
- 15.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. 2017. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650. ( 10.1038/nature22975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse SS, Mazet JAK, Woolhouse M, Parrish CR, Carroll D, Karesh WB, Zambrana-Torrelio C, Lipkin WI, Daszak P. 2012. Prediction and prevention of the next pandemic zoonosis. Lancet 380, 1956–1965. ( 10.1016/S0140-6736(12)61684-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes EC, Rambaut A, Andersen KG. 2018. Pandemics: spend on surveillance, not prediction. Nature 558, 180–182. ( 10.1038/d41586-018-05373-w) [DOI] [PubMed] [Google Scholar]
- 19.Karesh WB, et al. 2012. Ecology of zoonoses: natural and unnatural histories. Lancet 380, 1936–1945. ( 10.1016/S0140-6736(12)61678-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisson I-A, Ssebide BJ, Marra PP. 2015. Early detection of emerging zoonotic diseases with animal morbidity and mortality monitoring. EcoHealth 12, 98–103. ( 10.1007/s10393-014-0988-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson SH, et al. 2012. Dead or alive: animal sampling during Ebola hemorrhagic fever outbreaks in humans. Emerg. Health Threats J. 5, 9131–9139. ( 10.3402/ehtj.v5i0.9134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mbonye A, Wamala J, Nanyunja M, Opio A, Aceng J, Makumbi I. 2014. Ebola viral hemorrhagic disease outbreak in West Africa: lessons from Uganda. Afr. Health Sci. 14, 495–501. ( 10.4314/ahs.v14i3.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen KH, et al. 2016. Lessons from the Ebola outbreak: action items for emerging infectious disease preparedness and response. EcoHealth 13, 200–212. ( 10.1007/s10393-016-1100-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang L-Q, et al. 2016. Transmission dynamics of Ebola virus disease and intervention effectiveness in Sierra Leone. Proc. Natl Acad. Sci. USA 113, 4488–4493. ( 10.1073/pnas.1518587113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott J, Bushmaker T, Fischer R, Miazgowicz K, Judson S, Munster VJ. 2015. Postmortem stability of Ebola virus. Emerg. Infect. Dis. 21, 856–859. ( 10.3201/eid2105.150041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Wit E, et al. 2016. Ebola Laboratory response at the eternal love winning Africa Campus, Monrovia, Liberia, 2014–2015. J. Infect. Dis. 214(Suppl. 3), jiw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert SN, Schulz JE, Matson MJ, Bushmaker T, Marzi A, Munster VJ. 2018. Long-range polymerase chain reaction method for sequencing the Ebola virus genome from ecological and clinical samples. J. Infect. Dis. 218, S301–S304. ( 10.1093/infdis/jiy290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thumbi SM, Njenga MK, Otiang E, Otieno L, Munyua P, Eichler S, Widdowson M-A, McElwain TF, Palmer GH. 2019. Mobile phone-based surveillance for animal disease in rural communities: implications for detection of zoonoses spillover. Phil. Trans. R. Soc. B 374, 20190020 ( 10.1098/rstb.2019.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blow JA, Mores CN, Dyer J, Dohm DJ. 2008. Viral nucleid acid stabilisation by RNA extraction reagent. J. Virol. Methods 150, 41–44. ( 10.1016/j.jviromet.2008.02.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material. The figures were produced using QGIS 2.18 Las Palmas software (Free Software Foundation, Inc., 51 Franklin Street, Fifth Floor, Boston, MA, USA).