Figure 1. Trial Design and Randomization.
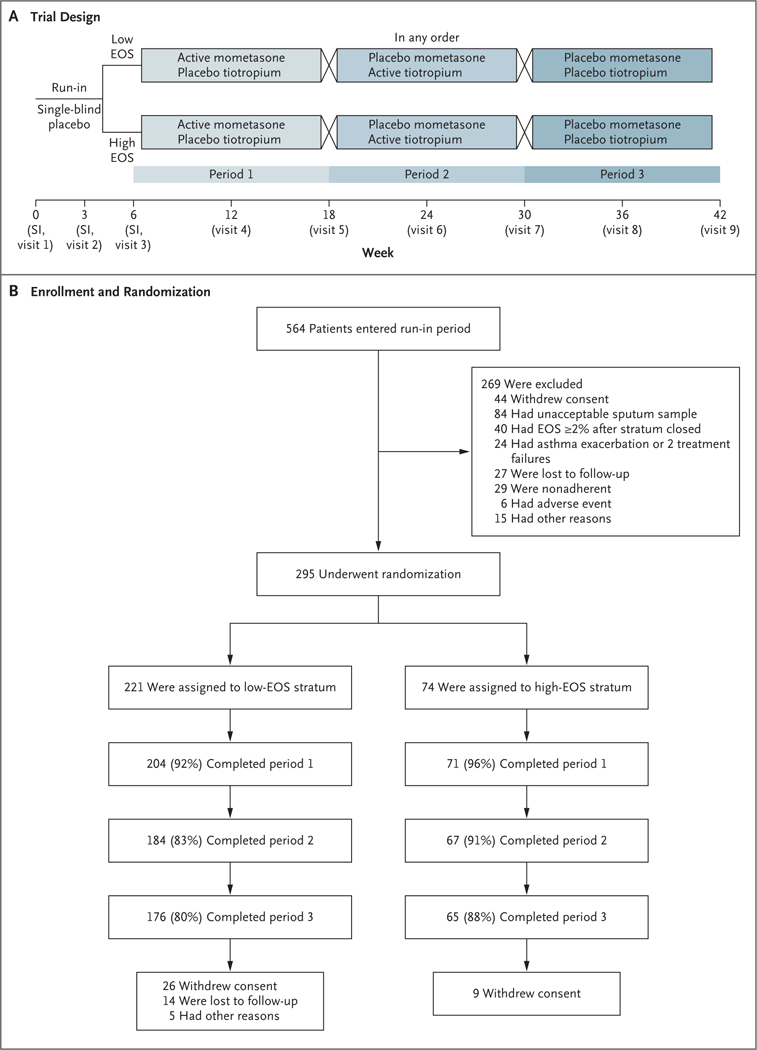
Panel A shows the trial design calling for the enrollment of patients who met the guideline criteria for step 2 asthma treatment in a 6-week single-blind placebo run-in period for characterization of asthma. Sputum induction (SI) was performed up to three times to guarantee the collection of two acceptable samples. At the end of the run-in period, patients who continued to meet the criteria for step 2 treatment and who met the adherence criteria for medication use and diary completion were stratified according to the sputum eosinophil (EOS) level (<2% or ≥2%) and were randomly assigned to receive one of three blinded regimens in random sequence for 12 weeks each. Throughout the trial, patients used an electronic diary to record asthma symptoms, nighttime awakenings, and morning and evening peak expiratory flow. Inhaler use was tracked by device dose counters. Panel B shows the number of patients who enrolled in the trial, underwent randomization, and completed the trial. A sputum sample was deemed to be unacceptable if it contained more than 80% squamous cells, if there was an inadequate sputum volume, or if the patient was unable to continue the induction procedure for at least 4 minutes. After 74 patients were categorized as being in the high-eosinophil stratum, subsequent patients with a high eosinophil level did not undergo randomization.
