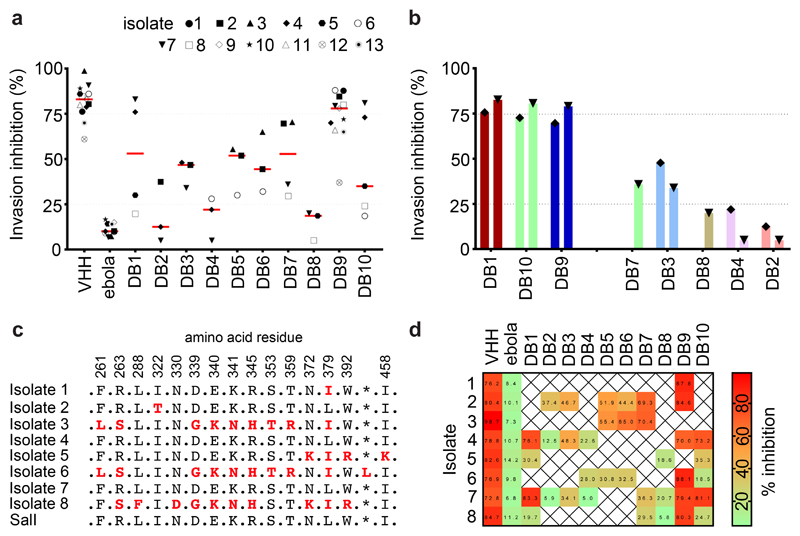
Figure 4. Inhibition of invasion of reticulocytes by Thai Plasmodium vivax clinical isolates.
(A) Plasmodium vivax ex-vivo invasion assays were performed with thirteen separate isolates of infected blood from local patients. Each data point represents the % inhibition of each antibody against one of the thirteen isolates. All antibodies were tested at a final concentration of 1 mg/mL, except the positive control anti-DARC VHH (VHH) which was assayed at 25 μg/mL. The red bars represent the median % inhibition for each antibody. A recombinant human IgG1 anti-Ebolavirus mAb (ebola) was used as a negative control at 1mg/mL. (B) Percentage invasion inhibition by mAbs tested against the two Thai Plasmodium vivax isolates which share the PvDBPII gene sequence of SalI; isolate #4 (left hand bar of each pair) and isolate #7 (right hand bar of each pair). This demonstrates the degree of correlation with the hierarchy of mAb inhibition in the transgenic Plasmodium knowlesi GIA assays (Figure 3A). (C) Amino acid polymorphisms found within the PvDBPII gene segment of the eight Thai Pv isolates for which we obtained sequence information. The vaccine homologous SalI reference sequence is shown in the bottom row. Amino acids that are the same as the reference sequence black, divergent residues are red and * represents absence of a leucine insertion between V429 and P430 in the SalI reference sequence. (D) Summary matrix showing the percentage inhibition of invasion by each mAb for each of the sequenced strains. The positive control was the camelid anti-DARC nanobody (VHH) at 25 μg/mL and the negative control was a recombinant human IgG1 anti-Ebolavirus mAb (ebola) at a concentration of 1 mg/mL.