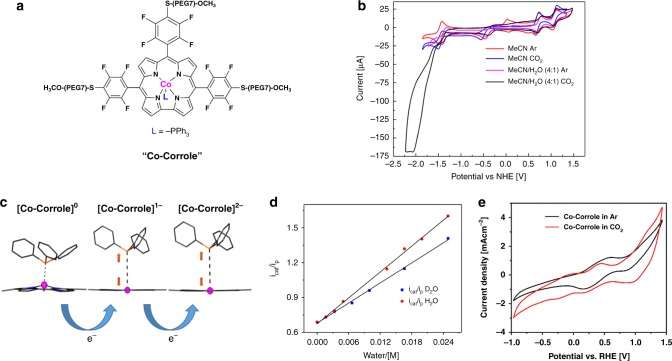
Fig. 1.
a Chemical structure of the Co-corrole. b Cyclic voltammetry of Co-corrole dissolved in CH3CN under Ar (red). Two metal centered redox peaks at −0.5 V (Co (III)/Co(II)) and −1.5 V (Co(II)/Co(I)) vs. NHE could be identified. The irreversibility of the redox peak at −0.5 V is likely due to the partial loss of the PPh3 ligand. Cyclic voltammetry of Co-corrole dissolved in CH3CN under CO2 (blue), in CH3CN/H2O (4:1) under Ar (pink) and in CH3CN/H2O (4:1) under CO2 (black). c DFT optimized geometries of [Co-corrole]0, 1e− and 2e− reduced species showing the movement of Co center into the central cavity of the corrole ring with concomitant lengthening of the Co-PPh3 bond upon successive reduction. d Kinetic isotpopic effect demonstrated by cyclic voltammetry of Co-Corrole (0.5 mM) recorded in CO2 saturated in acetonitrile in the presence of varying amount of H2O or D2O. Linear dependence of icat/ip on the concentration of water (analogous to plotting √kCO2 vs. [water], KIE = kCO2H/kCO2D = (SlopeH2O/slopeD2O)2 = (37.1057/28.7545)2 = 1.67). All cyclic voltammograms were recorded with 0.1 M TBAPF6 as supporting electrolyte using a glassy carbon as working electrode and a Ag/AgCl as reference electrode at a scan rate of 100 mV s−1. e Heterogeneous catalysis of 1 mM Co-corrole on carbon-fiber electrode under Ar (black) and CO2 (red) at pH = 6.0 (Ag/AgCl/KCl, Pt, 0.1 M NaClO4, 100 mV s−1)