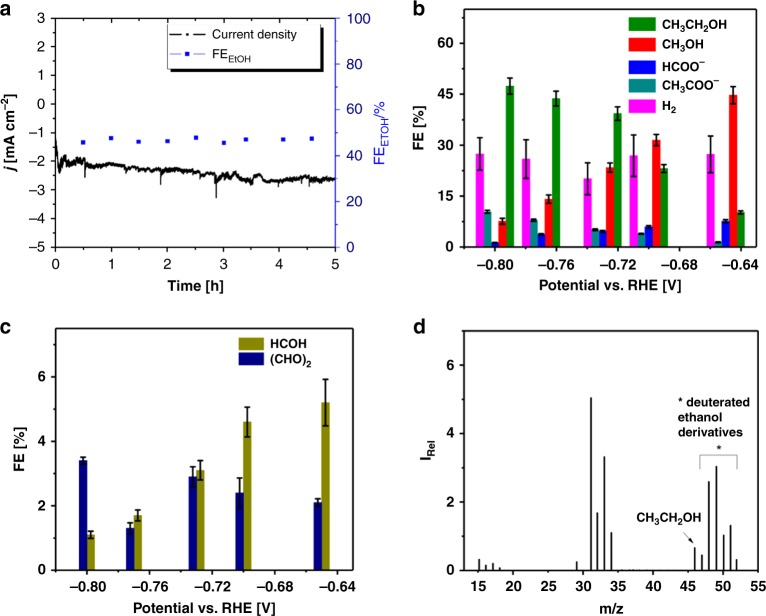
Fig. 2.
a Constant potential electrolysis of electrochemical CO2 reduction by the Co-corrole modified carbon paper electrode at a potential of −0.8 V vs. RHE (black curve), Faradaic efficiency for ethanol production over 5 h electrolysis (blue rectangles). b FE% vs. potential plot for potential dependent product formation. c FE% vs. potential plot for minor formed formaldehyde and solvated dimer of formaldehyde at different potentials. The error bars represent standard deviation of six measurements (three electrochemical reactions with two product analysis measurements for each reaction). d MS spectra obtained after electrolysis at −0.8 V vs. RHE in (1:3) D2O/H2O 0.1 M NaClO4 saturated with CO2