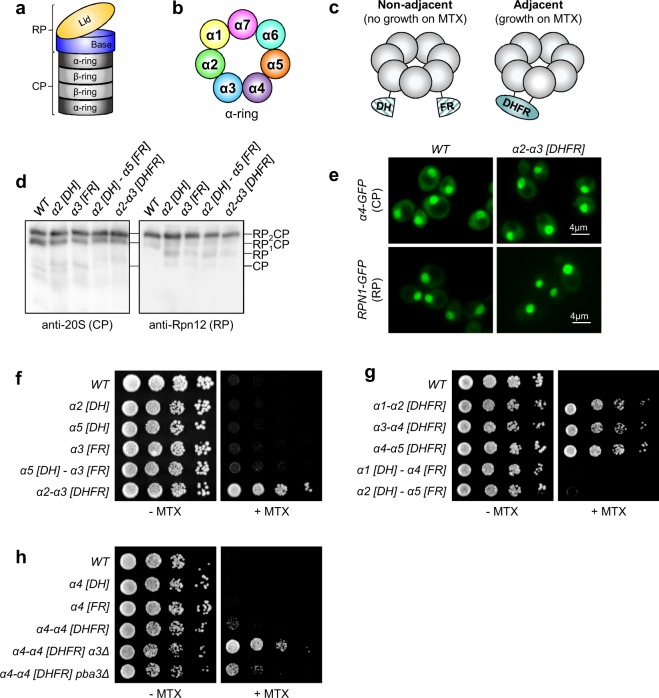
Figure 1.
Split-DHFR complementation reports on canonical and non-canonical CP subunit arrangements. (a) Illustration of the 26S proteasome depicting the major subcomplexes. RP, regulatory particle; CP, core particle. (b) Illustration of the canonical arrangement of α-subunits within the α-ring of the 20S core particle. (c) Schematic depicting split-DHFR complementation to monitor proteasome subunit juxtaposition in vivo. (d) Cell extracts from yeast strains expressing α-subunits fused with N- or C-terminal DHFR fragments (designated [DH] or [FR], respectively) from their chromosomal loci were separated by non-denaturing PAGE and immunoblotted with antibodies against the 20S CP (left) and the RP lid subunit Rpn12 (right). The positions of doubly-capped CP (RP2CP), singly-capped CP (RP1CP), RP, and CP are shown. Full-length blots are presented in Supplementary Fig. S8. (e) Subcellular localization of the CP subunit α4-GFP (top row) or RP subunit Rpn1-GFP (bottom row) is unaffected by expression of the α2-α3 [DHFR] reporter pair. (f–h) Equal numbers of cells from the indicated yeast strains were spotted in six-fold serial dilutions on synthetic complete plates lacking or containing methotrexate (MTX) and incubated for three days at 30 °C.