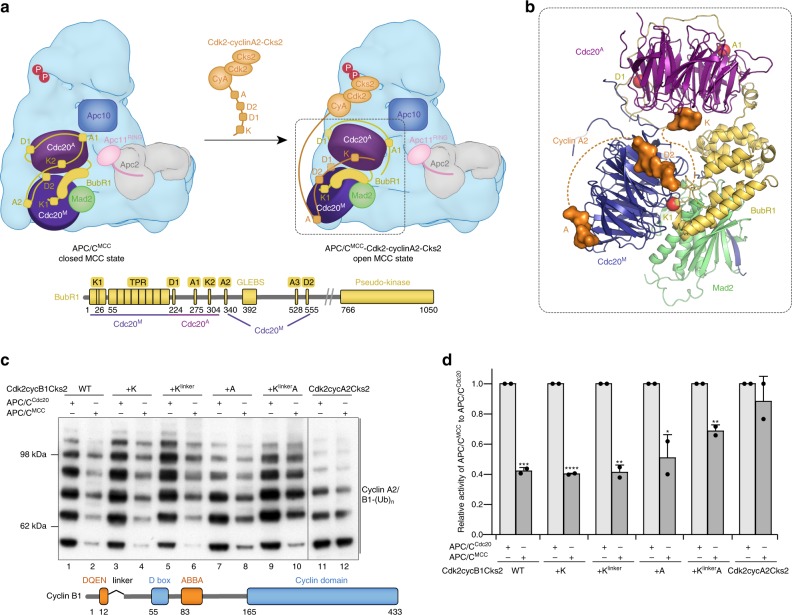
Fig. 7.
Mechanism of how cyclin A2 evades the SAC and conversion of cyclin B1 into a prometaphase substrate. a Schematic cartoon showing that Cdk-cyclinA2-Cks2 associates with APC/CMCC and displaces the ABBA motif (A2) and the D box (D2) of BubR1 from Cdc20M. The more exposed KEN-box binding site on Cdc20A is likely to accommodate the KEN box of cyclin A2. Binding of cyclin A2 may induce the open state MCC. We propose that the D2 box is the principle D box; however, the D1 box augments affinity through an avidity effect. Bottom: Schematic of BubR1 domain organization showing its degrons and the respective interacting Cdc20 subunits. b Close-up view of a model with cyclin A2 associating with Cdc20A-MCC (PDB 5LCW22). Cyclin A2 degrons are shown modelled as surface representations in orange occupying the more exposed degron-binding sites based on the equivalent pseudo-degrons of BubR122. The remaining three degron-binding sites where BubR1 degrons could still engage are highlighted as red spheres (A1, D1, K1). c, d Insertion of both the KEN box and ABBA motif into cyclin B1 allowed it to resist MCC-imposed inhibition (compare lanes 1, 2 and 9, 10). Cyclin B1 is in complex with Cdk2-Cks2. The histogram of relative APC/CMCC activity is normalized to respective APC/CCdc20 activity, with error bars indicating standard deviation, and significance is calculated using unpaired Student’s t-test (indicated with stars, n = 2, Supplementary Table 2). Source data are provided as a Source Data file