Abstract
Phylogenetic and taxonomic studies on the brown-rot fungi Postia and related genera, are carried out. Phylogenies of these fungi are reconstructed with multiple loci DNA sequences including the internal transcribed spacer regions (ITS), the large subunit (nLSU) and the small subunit (nSSU) of nuclear ribosomal RNA gene, the small subunit of mitochondrial rRNA gene (mtSSU), the translation elongation factor 1-α gene (TEF1), the largest subunit of RNA polymerase II (RPB1) and the second subunit of RNA polymerase II (RPB2). Ten distinct clades of Postia s.lat. are recognized. Four new genera, Amaropostia, Calcipostia, Cystidiopostia and Fuscopostia, are established, and nine new species, Amaropostia hainanensis, Cyanosporus fusiformis, C. microporus, C. mongolicus, C. piceicola, C. subhirsutus, C. tricolor, C. ungulatus and Postia sublowei, are identified. Illustrated descriptions of the new genera and species are presented. Identification keys to Postia and related genera, as well as keys to the species of each genus, are provided.
Keywords: Fomitopsidaceae, multi-marker analyses, Oligoporus, phylogeny, taxonomy, Tyromyces, wood-inhabiting fungi
INTRODUCTION
Postia was established by Fries (1874). Postia species are characterized by annual growth habit, mostly soft to corky fruiting bodies when fresh, a monomitic hyphal system with clamped generative hyphae, allantoid to cylindrical basidiospores which are usually thin-walled, negative in Melzer’s reagent and acyanophilous in Cotton Blue, and producing a brown rot (Hattori et al. 2011, Cui & Li 2012). More than 60 species have been accepted in the genus worldwide so far (Jülich 1982, Larsen & Lombard 1986, Renvall 1992, Buchanan & Ryvarden 2000, Wei & Dai 2006, Hattori et al. 2011, Dai 2012, Shen et al. 2015), of which 34 species were recorded from China (Wei & Qin 2010, Dai 2012, Shen et al. 2014, 2015).
Postia is closely related to Oligoporus and Spongiporus. Historically, most taxa in the three genera were placed in Tyromyces (Murrill 1907, 1912, Bondartsev & Singer 1941, Lowe 1975, Ryvarden 1981). However, it became clear that the species in Tyromyces cause a white rot, while species in the other three genera cause a brown rot (Gilbertson & Ryvarden 1987, Ryvarden 1991, Ryvarden & Gilbertson 1994). Because no species was listed when Postia was first proposed in Fries (1874), some mycologists did not accept Postia, but supported Spongiporus or Oligoporus instead. Oligoporus was established in 1888 by Brefeld and included three species initially, with the characteristics of fleshy fruitbody when fresh, turning to fragile when dry and allantoid to cylindrical basidiospores. Later, Gilbertson & Ryvarden (1985) placed 22 taxa into Oligoporus containing two previous species in Tyromyces and gradually Oligoporus was widely used (Gilbertson & Ryvarden 1987, Ryvarden & Gilbertson 1994, Núñez & Ryvarden 2001, Bernicchia 2005, Ryvarden & Melo 2014). Murrill erected 29 genera, including Spongiporus for North American polypores in early 20th century, and he defined Spongiporus species as brown rot fungi with whitish and spongiose basidiocarps that bear cylindrical basidiospores. David (1980) transferred 13 Tyromyces species into Spongiporus, adopted by many other studies (Bondartsev & Singer 1941, Lowe 1975, Ryvarden 1981). In fact, Postia is the oldest name among the competing genera. Some mycologists combined the brown rot taxa of Tyromyces into Postia (Renvall 1992, Niemelä et al. 2005, Wei & Dai 2006, Hattori et al. 2011, Cui & Li 2012, Pildain & Rajchenberg 2013). With more species recognized in Postia, the definitions of the genus and related genera remain murky, and so are the genetic relationships among these fungi.
Pildain & Rajchenberg (2013) sequenced the ITS and nLSU regions from eleven species of Postia and related species; their phylogenetic analysis indicated that most species in Postia and Oligoporus were monophyletic, but supported the transfer of P. placenta into its own genus as Rhodonia placenta, in agreement with previous studies (Boidin et al. 1998, Kim et al. 2001, Binder et al. 2005, Niemelä et al. 2005). Ortiz-Santana et al. (2013) investigated the phylogenetic relationships among members of the antrodia clade with molecular data from ITS and nLSU regions; in their study, species of Postia s.lat. were divided into four clades: the Spongiporus clade, the Oligoporus clade, the Postia s.str. clade and the Spongiporus undosus clade. Cui et al. (2014) discussed the phylogenetic position of the monotypic genus Osteina in the Fomitopsidaceae of Polyporales, and accepted Osteina obducta rather than Oligoporus obductus.
Up to now, no comprehensive investigation has been carried out on Postia s.lat. with sufficient sampling, and taxonomic delimitation of Postia s.lat. has been controversial and remained insufficiently resolved (Donk 1960, Larsen & Lombard 1986, Ryvarden 1991, Walker 1996, Pildain & Rajchenberg 2013). In this study, we carried out further taxonomic studies and phylogenetic analyses by sampling more species to clarify the relationships of Postia and related genera including Oligoporus, Osteina, Rhodonia and Spongiporus.
MATERIALS AND METHODS
Morphological studies
Most of the studied specimens were deposited at the herbaria of the Institute of Microbiology, Beijing Forestry University (BJFC), the Institute of Applied Ecology, Chinese Academy of Sciences (IFP), the private herbarium of Dr. J. Vlasák in Czech Republic (JV), the Botanical Museum of the University of Oslo, Norway (O), Université Claude Bernard, France (LY), Botanical Museum of University of Helsinki, Finland (H), Royal Botanic Gardens, Kew, UK (K) and the Pennsylvania State University, USA (PAC). Macro-morphological descriptions were based on the field notes and the herbarium specimens. Colour terms followed Petersen (1996). Micro-morphological data were obtained from the dried specimens, and observed under a light microscope following Li et al. (2014) and Zhou et al. (2016). Sections were studied at a magnification of up to ×1000 using a Nikon Eclipse 80i microscope and phase contrast illumination. Drawings were made with the aid of a drawing tube. Microscopic features, measurements and drawings were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Spores were measured from sections cut from the tubes. In presenting the variation of spore size, 5 % of the measurements were excluded from each end of the range, and were given in parentheses. The following abbreviations were used: KOH = 5 % potassium hydroxide, CB = Cotton Blue, CB+ = cyanophilous, CB– = acyanophilous, IKI = Melzer’s reagent, IKI– = neither amyloid nor dextrinoid, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = variation in the L/W ratios between the specimens studied, n (a/b) = number of spores (a) measured from given number (b) of specimens.
Phylogenetic analyses
A CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd, Beijing) was used to extract total genomic DNA from dried specimens, and performed the polymerase chain reaction (PCR) according to the manufacturer’s instructions (Chen et al. 2016). The ITS region was amplified with primer pairs ITS5 and ITS4 (White et al. 1990). The nLSU region was amplified with primer pairs LR0R and LR7 (https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/). The mtSSU region was amplified with primer pairs MS1 and MS2 (White et al. 1990). The nSSU regions were amplified with primer pairs NS1 and NS4 (White et al. 1990). Part of TEF1 was amplified with primer pairs EF1-983F and EF1-1567R (Rehner & Buckley 2005). The RPB1 was amplified with primer pairs RPB1-Af and RPB1-Cf (Matheny et al. 2002). RPB2 was amplified with primer pairs fRPB2-f5F and bRPB2-7.1R (Matheny 2005). The PCR procedure for ITS and mtSSU was as follows: initial denaturation at 95 °C for 3 min, followed by 34 cycles at 94 °C for 40 s, 54 °C for ITS and 55 °C for mtSSU for 45 s and 72 °C for 1 min, and a final extension of 72 °C for 10 min. The PCR procedure for nLSU, nSSU and TEF1 was as follows: initial denaturation at 94 °C for 1 min, followed by 34 cycles at 94 °C for 30 s, 50 °C for nLSU and 59 °C for TEF1 for 1 min and 72 °C for 1.5 min, and a final extension of 72 °C for 10 min. The PCR procedure for RPB1 and RPB2 follow Justo & Hibbett (2011) with slight modifications: initial denaturation at 94 °C for 2 min, followed by 10 cycles at 94 °C for 40 s, 60 °C for 40 s and 72 °C for 2 min, then followed by 37 cycles at 94 °C for 45 s, 55 °C for 1.5 min and 72 °C for 2 min, and a final extension of 72 °C for 10 min. The PCR products were purified and sequenced at Beijing Genomics Institute. All newly generated sequences were deposited at GenBank (Table 1).
Table 1.
A list of species, specimens and GenBank accession numbers of sequences used in this study.
a Newly generated sequences for this study
The sequenced dataset included 112 Postia s.lat. samples, of which 83 were types, paratypes or specimens from type localities. We used sequences mainly based on specimens from China, because those specimens were identified with careful morphological examinations and had more complete multi-gene sequence fragments. Additional sequences were downloaded from GenBank (Table 1) and were mainly referred to Ortiz-Santana et al. (2013) and Han et al. (2016). All sequences were aligned using ClustalX (Thompson et al. 1997) and manually adjusted in BioEdit (Hall 1999). The final concatenated sequence alignment was deposited in TreeBase (https://www.treebase.org/treebase-web/search/studySearch.html; submission ID 21389).
Most parsimonious phylogenies were inferred from the combined 3-gene dataset (ITS+nLSU+RPB2) and 7-gene dataset (ITS+nLSU+nSSU+mtSSU+TEF1+RPB1+RPB2), and their congruences were evaluated with the incongruence length difference (ILD) test (Farris et al. 1994) implemented in PAUP* v. 4.0b10 (Swofford 2002), under heuristic search and 1 000 homogeneity replicates. Settings for phylogenetic analyses followed Song et al. (2016) and Zhao et al. (2015). Sequences of Trametes suaveolens and Coriolopsis polyzona obtained from GenBank were used as outgroups to root trees following Binder et al. (2013) and Han et al. (2016). Maximum parsimony (MP) analysis was applied to the combined multiple genes dataset, this test under heuristic search and 1 000 homogeneity replicates gave a P value of 1.000, much greater than 0.01, which meant there was no discrepancy among the seven loci in reconstructing phylogenetic trees. The tree construction procedure was performed in PAUP* v. 4.0b10. All characters were equally weighted and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1 000 random sequence additions. Max-trees were set to 5 000, branches of zero length were collapsed and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BT) analysis with 1 000 replicates (Felsenstein 1985). Descriptive tree statistics tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for each most parsimonious tree (MPT) generated. RAxML v. 7.2.8 was used to construct a maximum likelihood (ML) tree with GTR+G+I model of site substitution (Stamatakis 2006). The branch support was evaluated with bootstrapping method of 1 000 replicates (Hillis & Bull 1993). Phylogenetic trees were visualized using Treeview (Page 1996).
MrModeltest v. 2.3 (Posada & Crandall 1998, Nylander 2004) was used to determine the best-fit evolution model for the combined multi-gene dataset for Bayesian inference (BI). Bayesian inference was calculated with MrBayes v. 3.1.2 with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites (Ronquist & Huelsenbeck 2003). Four Markov chains were run for 2 runs from random starting trees for 5 million generations (3-gene dataset), for 10 million generations (7-gene dataset) until the split deviation frequency value < 0.01, and sampled every 100th generation. A majority rule consensus tree of all remaining trees was calculated. Branches that received bootstrap ≥ 75 % (MP and BS) and Bayesian posterior probabilities (BPP) ≥ 0.95 were considered as significantly supported, respectively.
RESULTS
Phylogenetics analyses
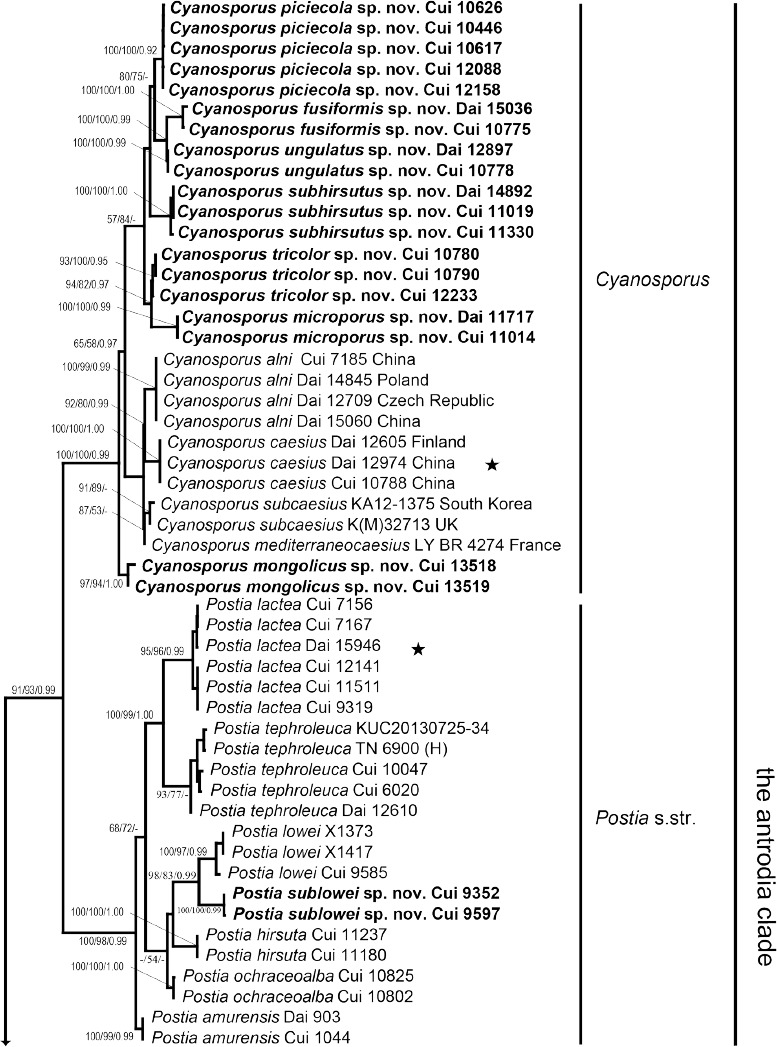
The combined 3-gene dataset included sequences from 176 fungal samples representing 91 taxa. The dataset had an aligned length of 2 937 characters, of which 1 337 characters were constant, 268 were variable and parsimony-uninformative and 1 332 were parsimony-informative. Maximum parsimony analysis yielded 153 equally parsimonious trees (TL = 10077, CI = 0.286, RI = 0.751, RC = 0.214, HI = 0.714). Best model for the combined 3-gene dataset estimated and applied in the Bayesian analysis was GTR+I+G. The average standard deviation of split frequencies in the Bayesian analysis reached 0.008252. Bayesian analysis and ML analysis resulted in a similar topology as MP analysis, and the MP tree inferred from the combined 3-gene dataset was shown in Fig. 1. The phylogeny (Fig. 1) inferred from the combined 3-gene sequences demonstrated 42 major lineages (including four new genera) for the sampled 89 species of the antrodia clade, and confirmed Postia s.lat. is polyphyletic.
Fig. 1.
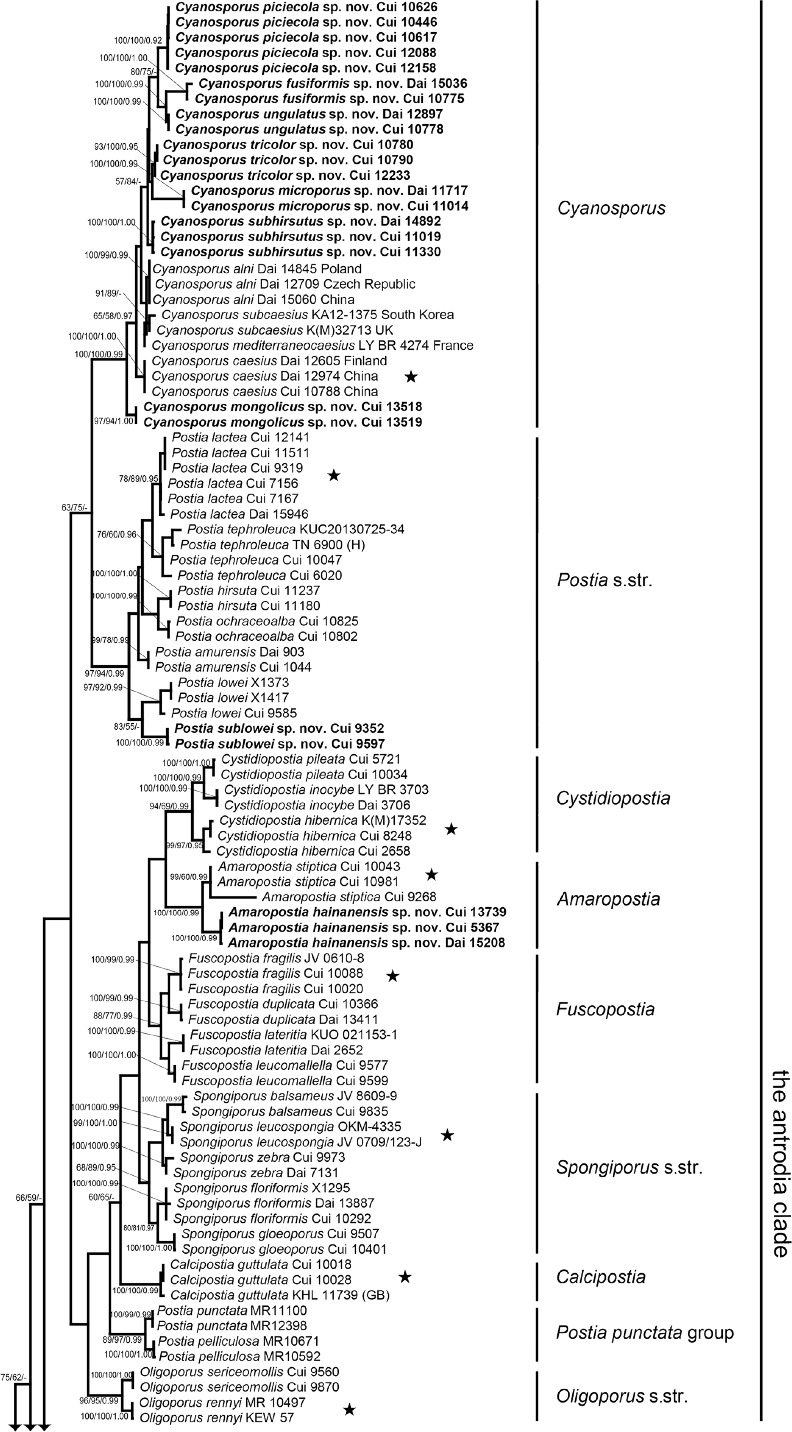
Maximum parsimony tree illustrating the phylogeny of Postia and its related genera in the antrodia clade based on the combined sequences dataset of ITS+nLSU+RPB2. Branches are labelled with parsimony bootstrap proportions > 50 %, maximum likelihood bootstrap > 50 %, and Bayesian posterior probabilities > 0.95. ★ = generic type.
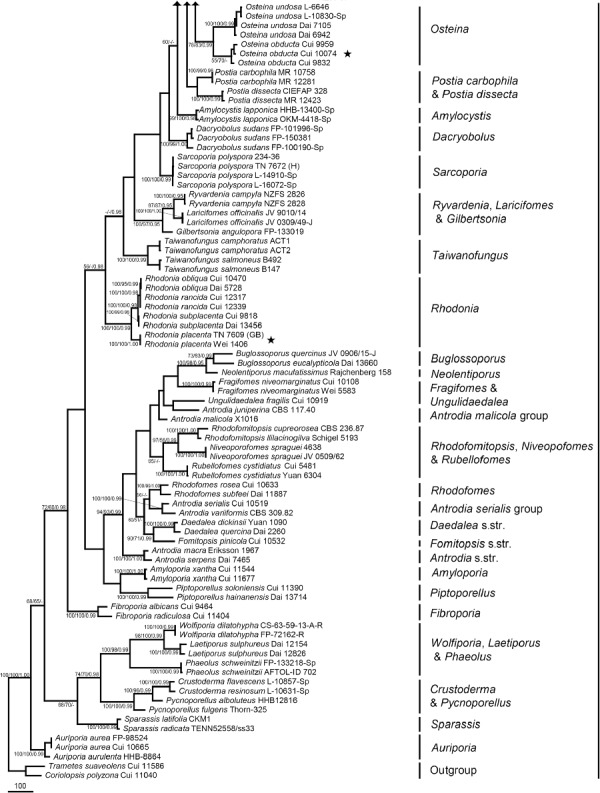
The combined 7-gene dataset included 129 fungal samples representing 53 taxa. The dataset had an aligned length of 6 428 characters, of which 4 145 characters were constant, 256 were variable and parsimony-uninformative and 2 027 were parsimony-informative. Maximum parsimony analysis yielded 12 equally parsimonious trees (TL = 7946, CI = 0.456, RI = 0.825, RC = 0.377, HI = 0.544). Best model for the combined 7-gene dataset estimated and applied in the Bayesian analysis was GTR+I+G with equal frequency of nucleotides. Bayesian analysis and ML analysis resulted in a similar topology as MP analysis, and the MP tree inferred from the combined 7-gene dataset was shown in Fig. 2. The 7-gene based phylogeny demonstrated that 41 species previously belonging to Postia s.lat. were embedded in ten lineages (Fig. 2).
Fig. 2.
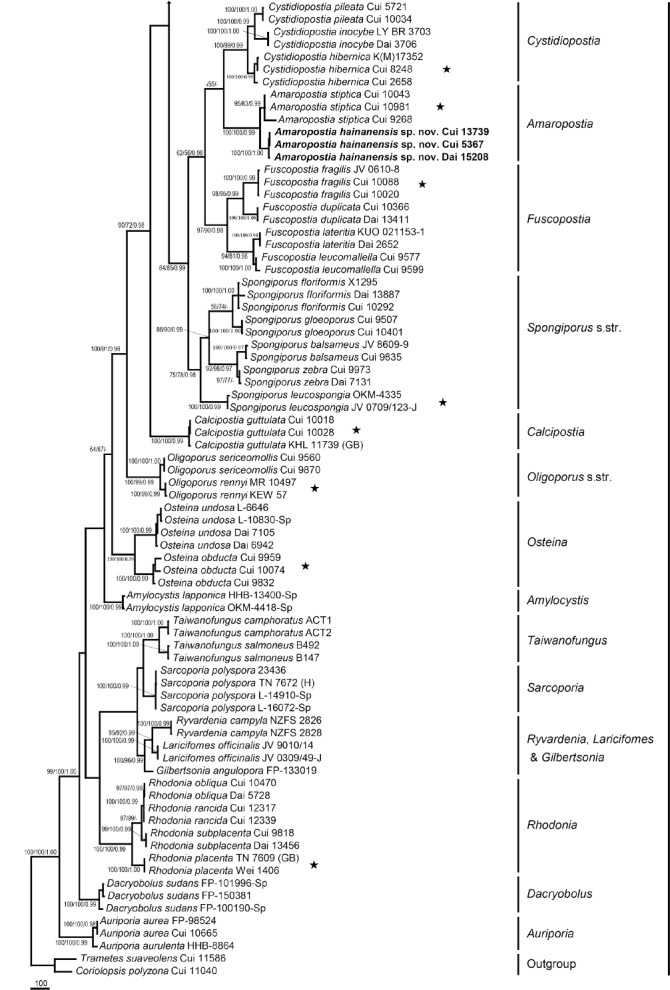
Maximum parsimony tree illustrating the phylogeny of Postia and its related genera in the antrodia clade based on the combined sequences dataset of ITS+nLSU+nSSU+mtSSU+TEF1+RPB1+RPB2. Branches are labelled with parsimony bootstrap proportions > 50 %, maximum likelihood bootstrap > 50 %, and Bayesian posterior probabilities > 0.95. ★ = generic type.
Taxonomy
Amaropostia B.K. Cui, L.L. Shen & Y.C. Dai, gen. nov. — MycoBank MB819256
Type species. Amaropostia stiptica (Pers.) B.K. Cui, L.L. Shen & Y.C. Dai.
Etymology. Amaropostia (Lat.) refers to the new genus resembling Postia but with bitter taste.
Diagnosis. Morphologically, Amaropostia differs from Postia s.str. by woody hard basidiocarps when dry, relatively small pores, bitter taste, and cylindrical basidiospores.
Basidiocarps annual, sessile, soft corky when fresh, woody hard when dry, taste bitter. Pileal surface white when fresh, cream to buff when dry, glabrous, azonate. Pore surface white when fresh, cream or with yellowish tint upon drying; pores small, round to angular. Context white, woody hard. Tubes white to cream, brittle. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Cystidia absent, fusoid cystidioles occasionally present. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–.
Amaropostia hainanensis B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank Mb819258; Fig. 3a, 4
Fig. 3.

Basidiocarps of the new species. a. Amaropostia hainanensis (Cui 13739); b. Cyanosporus fusiformis (Dai 15036); c. Cyanosporus microporus (Cui 11014); d. Cyanosporus mongolicus (Cui 13518); e. Cyanosporus piceicola (Cui 10626); f. Cyanosporus subhirsutus (Dai 14892); g. Cyanosporus tricolor (Cui 12233); h. Cyanosporus ungulatus (Dai 12897). i. Postia sublowei (Cui 9597). — Scale bars: a = 0.5 cm; b–i = 1 cm.
Fig. 4.
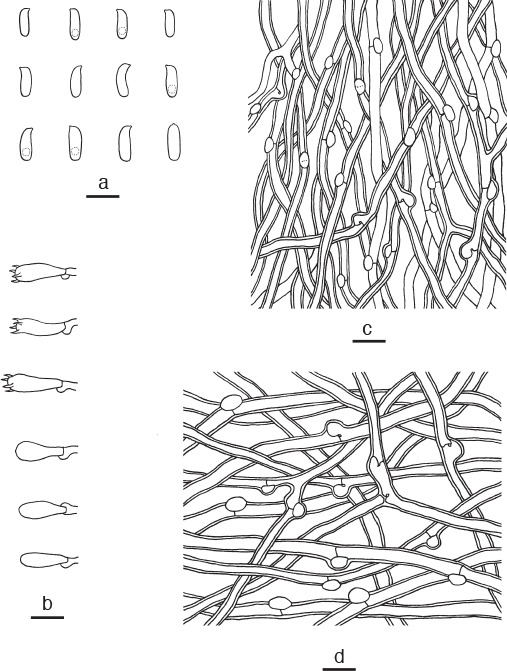
Microscopic structures of Amaropostia hainanensis. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Hainan Province, Ledong County, Jianfengling Forest Park, on fallen angiosperm branch, 21 Nov. 2015, B.K. Cui, Cui 13739 (BJFC).
Etymology. Hainanensis (Lat.) refers to the type locality, Hainan Province of China.
Diagnosis. Amaropostia hainanensis differs from other species in the genus by shell-shaped pileus, small angular pores, and slightly curved cylindrical basidiospores.
Basidiome annual, sessile, solitary, soft and watery when fresh, becoming corky to woody hard upon drying, taste bitter; pileus shell-shaped, projecting up to 2 cm, 2.5 cm wide and 0.8 cm thick at base. Pileal surface white when fresh, glabrous, becoming cream to buff; margin acute, concolorous with pileal surface. Pore surface white when fresh, becoming buff when dry; sterile margin narrow to almost lacking, white when fresh, becoming clay-buff upon drying; pores angular, 7–9 per mm; dissepiments thin, entire. Context white, woody hard, up to 0.5 cm thick. Tubes white, more or less brittle, up to 0.3 cm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thick-walled with a wide lumen, occasionally branched, interwoven, 3–6 μm diam. Generative hyphae in trama hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2–3 μm diam. Cystidia or cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 12.5–14 × 4–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores cylindrical, slightly curved, hyaline, thin-walled, smooth, usually bearing a guttule and tapering at apiculus, IKI–, CB–, 4(–4.5)–5.5(–6) × 1.5–2 μm, L = 4.53 μm, W = 1.68 μm, Q = 2.59–2.73 (n = 90/3).
Additional specimens (paratypes) examined. China, Hainan Province, Lingshui County, Diaoluoshan Forest Park, on fallen angiosperm branch, 22 Nov. 2007, B.K. Cui, Cui 5367 (BJFC 003408); Qiongzhong County, Limushan Forest Park, on fallen angiosperm branch, 30 May 2015, Y.C. Dai, Dai 15208 (BJFC 019319).
Notes — Amaropostia hainanensis and A. stiptica have annual, pileate basidiocarps with glabrous pileal surface and white to buff pore surface, bitter taste, and similar cylindrical basidiospores, but A. stiptica has bigger pores (5–6 per mm) and fusoid cystidioles (Ryvarden & Melo 2014). In addition, A. stiptica is widespread in coniferous forests in boreal temperate areas, while A. hainanensis is only found in tropical areas of South China.
Amaropostia stiptica (Pers.) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank Mb819257
Basionym. Boletus stipticus Pers., Syn. Meth. Fung. 2: 525. 1801.
= Oligoporus stipticus (Pers.) Gilb. & Ryvarden, N. Amer. Polyp. 2: 485. 1987.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Picea, 9 Aug. 2011, B.K. Cui, Cui 10043 (BJFC 010936); Shandong Province, Taian, Taishan, on fallen trunk of Pinus, 4 Aug. 2012, B.K. Cui, Cui 10981 (BJFC 013903); Xizang Autonomous Region (Tibet), Linzhi County, Lulang, on fallen trunk of Pinus, 16 Sept. 2010, B.K. Cui, Cui 9268 (BJFC 008207). – Czech Republic, Libochovka, Hluboká, on Picea, J. Vlasák 8911/27 (JV). – Finland, Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Picea, 22 Sept. 2010, Y.C. Dai, Dai 11797 (BJFC 008904). – Norway, Oslo, Botanical Garden, on stump of Populus, 9 Nov. 2011, Y.C. Dai, Dai 12677 (BJFC 012261). – USA, Pennsylvania, on Picea, J. Vlasák 0407/32 (JV).
Notes — Oligoporus stipticus is characterized by woody hard basidiocarps when dry, glabrous pileal surface, bitter taste, fusoid cystidioles, and cylindrical basidiospores. It usually grows on coniferous woods and is widely distributed in temperate areas. Although we did not find the type specimen, we have examined the specimens from China, Europe and North America. The morphological characters of all the studied specimens fit well with Oligoporus stipticus. Based on morphological characters and phylogenetic analyses, we transferred Oligoporus stipticus to Amaropostia as a new combination. For a detailed description of the species, see Oligoporus stipticus by Ryvarden & Melo (2014).
Calcipostia B.K. Cui, L.L. Shen & Y.C. Dai, gen. nov. — MycoBank MB819259
Type species. Calcipostia guttulata (Sacc.) B.K. Cui, L.L. Shen & Y.C. Dai.
Etymology. Calcipostia (Lat.) refers to the new genus resembling Postia but with calcareous basidiocarps and circular guttulate depressions attached to the pileal surface.
Diagnosis. Morphologically, Calcipostia differs from Postia s.str. by big basidiocarps with calcareous texture, circular guttulate depressions on the pileal surface, bitter taste, and short-cylindrical to oblong basidiospores.
Basidiocarps annual, pileate or laterally substipitate. Pileus fleshy when fresh, fragile to hard fibrous when dry. Pileal surface white when fresh, buff or pale brown when dry, with circular guttulate depressions. Pore surface white to cream when fresh, pale buff when dry; pores round to angular. Context white to cream, hard fibrous. Tubes cream, fragile. Taste slightly bitter. Hyphal system monomitic, generative hyphae with clamp connections, IKI–, CB–. Cystidia absent; fusoid cystidioles present. Basidiospores short-cylindrical to oblong, hyaline, thin-walled, smooth, IKI–, CB–.
Calcipostia guttulata (Sacc.) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819261
Basionym. Polyporus guttulata Sacc., Syll. Fung. 6: 106. 1888.
= Oligoporus guttulatus (Peck) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Abies, 9 Aug. 2011, B.K. Cui, Cui 10018 (BJFC 010911), Cui 10028 (BJFC 010921); Xizang Autonomous Region (Tibet), Linzhi County, on fallen trunk of Abies, 18 Sept. 2010, B.K. Cui, Cui 9444 (BJFC 008382). – Finland, Etelä-Häme, Padasjoki Strict Nature Reserve, on fallen trunk of Picea, 10 Oct. 1992, Y.C. Dai, Dai 238 (BJFC 002076). – Poland, Opole, Stawmatmloy, on fallen trunk of Fagus, 4 Oct. 2014, Y.C. Dai, Dai 14864 (BJFC 017977). – USA, New York, on Picea, J. Vlasák 0509/189 (JV).
Notes — This species is characterized by pileate basidiocarps with calcareous texture, circular guttulate depressions on the pileal surface, bitter taste, and short-cylindrical to oblong basidiospores. This species is widely distributed in temperate areas and usually grows on coniferous woods. It was originally described from the USA; although we did not find the type specimen, we have examined the specimens from China, Europe and USA. In addition, the older specimens deposited at the herbaria of BPI, NY, NYS and SYRF have been extensively studied by Lowe (1975). The morphological characters of our studied specimens fit well with the descriptions of Lowe (1975) and Ryvarden & Melo (2014). Based on morphological characters and phylogenetic analyses, we transferred Oligoporus guttulatus to Calcipostia as a new combination. For a detailed description of the species, see Oligoporus guttulatus by Ryvarden & Melo (2014).
Cyanosporus McGinty, Mycol. Notes 33: 436. 1909. — MycoBank MB819263
Type species. Cyanosporus caesius (Schrad.) McGinty.
Basidiocarps annual, resupinate to effused-reflexed or pileate, soft corky when fresh, corky to fragile when dry. Pileal surface white, cream, buff, yellow to greyish, usually with blue tint when fresh, cream, grey to greyish brown when dry, velutinate to hirsute or glabrous. Pore surface white to cream, frequently bluish when bruised, pores round to angular. Context white to cream, corky. Tubes cream, fragile. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Cystidia usually absent, gloeocystidia and thin-walled cystidioles occasionally present. Basidiospores narrow, allantoid to cylindrical, hyaline, usually slightly thick-walled, smooth, IKI–, weakly CB+.
Notes — The name Cyanosporus was proposed in 1909 as a monotypic genus for Polyporus caesius (McGinty 1909), but it was not accepted in subsequently studies (Donk 1960, Jahn 1963, Lowe 1975). Then the Postia caesia complex was mentioned based on recent molecular phylogenetic studies in which the Postia caesia complex formed a distinctive morphological group within the genus (Ţura et al. 2008). Papp (2014) proposed the combination Postia subg. Cyanosporus for the Postia caesia complex including five species (P. alni, P. caesia, P. luteocaesia, P. mediterraneocaesia, P. subcaesia). In our study, the genus Cyanosporus is supported as an independent genus which contains the Postia caesia complex and seven other clearly distinguished new species from China. Phylogenetically, the new species are closely related to the Postia caesia complex; all the species in the complex form a well-supported lineage (Fig. 1, 2), which is distant from Postia s.str. Morphologically, Cyanosporus differs from Postia s.str. by its more or less bluish basidiocarps, usually narrow allantoid, thin- to slightly thick-walled and weakly cyanophilous basidiospores.
Cyanosporus alni (Niemelä & Vampola) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819264
Basionym. Postia alni Niemelä & Vampola, Karstenia 41: 7. 2001.
= Oligoporus alni (Niemelä & Vampola) Piątek, Polish Bot. J. 48: 17. 2003.
Specimens examined. China, Guizhou Province, Suiyang County, Kuankuoshui Nature Reserve, on fallen angiosperm trunk, 26 June 2014, Y.C. Dai, Dai 15060 (BJFC 018172); Hebei Province, Xinglong County, Wulingshan Nature Reserve, on fallen angiosperm trunk, 29 Aug. 2009, B.K. Cui, Cui 7185 (BJFC 005672). – Czech Republic, Ceske Budejovice, on fallen trunk of Fagus, 22 Nov. 2011, Y.C. Dai, Dai 12709 (BJFC 012293). – Finland, Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Populus, 15 Sept. 1997, Y.C. Dai, Dai 2652 (IFP 005405); on fallen trunk of Populus, 4 Nov. 2011, Y.C. Dai, Dai 12641 (BJFC 012223). – Poland, Brynica, Mcrow, on fallen trunk of Fagus, 3 Oct. 2014, Y.C. Dai, Dai 14845 (BJFC 017959). – Slovakia, Bratislava, on Alnus, 12 Oct. 1995, Vampola 32595 (holotype, H).
Notes — We have examined the type specimen and other specimens from China and Europe. Based on morphological characters and phylogenetic analyses, we transferred Postia alni to Cyanosporus as a new combination. For a detailed description of Cyanosporus alni, see Postia alni by Niemelä et al. (2001).
Cyanosporus caesius (Schrad.) McGinty, Mycol. Notes 33: 436. 1909
Basionym. Boletus caesius Schrad., Spic. Fl. Germ. 1: 167. 1794.
= Oligoporus caesius (Schrad.) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Picea, 20 Oct. 2012, B.K. Cui, Cui 10788 (BJFC 013710); Puge County, Luoji Mountain, on fallen trunk of Picea, 19 Sept. 2012, Y.C. Dai, Dai 12974 (BJFC 13220). – Finland, Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Picea, 3 Nov. 2011, Y.C. Dai, Dai 12605 (BJFC 012192). – Germany, Göttingen, on Picea, 27 Sept. 2012, LY BR 6776 (LY). – Spain, Cadiz Province, Sierra Grazalema Natural Park, on fallen trunk of Abies, 22 Nov. 2005, Y.C. Dai, Dai 7438 (BJFC 002060).
Notes — Karsten (1881) transferred Boletus caesius into Postia; later McGinty (1909) established Cyanosporus with C. caesius as the type species but this was not widely accepted. Then Spongiporus and Oligoporus were sequentially erected, and Cyanosporus caesius was accordingly treated as Spongiporus caesius and Oligoporus caesius, respectively. In our study, Cyanosporus was treated as an independent genus and C. caesius is one species in the Cyanosporus lineage (Fig. 1, 2). This species is common in Europe, North America and East Asia. For a detailed description, see Oligoporus caesius by Ryvarden & Melo (2014).
Cyanosporus fusiformis B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank Mb819269; Fig. 3b, 5
Fig. 5.
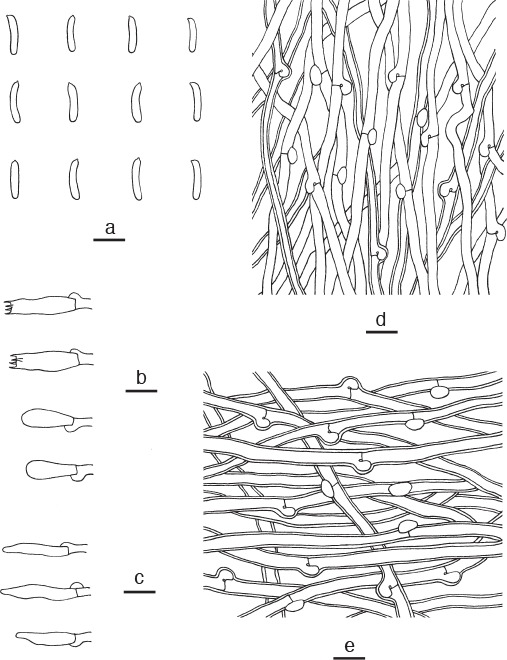
Microscopic structures of Cyanosporus fusiformis. a. Basidiospores; b. basidia and basidioles; c. cystidioles; d. hyphae from trama; e. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–e = 10 μm.
Holotype. China, Guizhou Province, Suiyang County, Kuankuoshui Nature Reserve, on dead angiosperm tree, 26 Nov. 2014, Y.C. Dai, Dai 15036 (BJFC 018149).
Etymology. Fusiformis (Lat.) refers to the fusiform cystidioles.
Diagnosis. Cyanosporus fusiformis differs from other species in the genus by semicircular pileus, fusiform cystidioles presenting in hymenium and slim allantoid basidiospores.
Basidiome annual, pileate or effused reflexed, solitary or imbricate, soft corky and without odour or taste when fresh, becoming hard corky to brittle upon drying. Pileus semicircular, projecting up to 1 cm, 1.2 cm wide and 3 mm thick at base. Pileal surface white to cream, with blue tint at centre when fresh, finely tomentose, becoming vinaceous grey to dark grey upon drying; margin acute, concolorous with pileal surface. Pore surface white when fresh, becoming clay-buff when dry; sterile margin almost lacking; pores round, 4–5 per mm; dissepiments thin, entire to lacerate. Context white, hard corky, up to 2 mm thick. Tubes white, brittle, up to 1 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, slightly thick-walled with a wide lumen, rarely branched, loosely interwoven, 3–5 μm diam. Generative hyphae in trama hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, loosely interwoven, 2–4 μm diam. Cystidia absent; cystidioles present, fusiform, hyaline, thin-walled, 10–13 × 3–5 μm. Basidia clavate, constricted at middle, bearing four sterigmata and a basal clamp connection, 12–15 × 4.5–6 μm; basidioles clavate, and distinctly smaller than basidia. Basidiospores slim allantoid, hyaline, thin-walled, smooth, IKI–, CB–, 4.5–5.2(–5.5) × 0.8–1.1 μm, L = 5.01 μm, W = 0.92 μm, Q = 5.21–5.45 (n = 60/2).
Additional specimen (paratype) examined. China, Sichuan Province, Luding County, Hailuogou Forest Park, on dead tree of Rhododendron, 20 Oct. 2012, B.K. Cui, Cui 10775 (BJFC 013697).
Notes — Two species of Cyanosporus produce cystidioles: C. fusiformis and C. mongolicus, but the latter one has resupinate basidiocarps and wider basidiospores (4.5–5 × 1.5–1.9 μm). In addition, its hyphae became swollen in KOH.
Cyanosporus luteocaesius (A. David) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank Mb819270
Basionym. Spongiporus luteocaesius A. David, Bull. Mens. Soc. Linn. Lyon 49: 119. 1980.
= Oligoporus luteocaesius (A. David) Ryvarden & Gilb., Syn. Fungorum 7: 421. 1993.
Specimens examined. France, Var, Massif des Maures, on Pinus, 26 Dec. 1970, David 929 (holotype, LY); Porquerolles, on Pinus, 13 Nov. 2004, LY BR 2605 (LY).
Notes — Spongiporus luteocaesius is a rare European species that exclusively grows on Pinus. We have examined the type specimen and another specimen from France. It has the typical morphological features of the Cyanosporus caesius group with blue greyish discoloration and similar allantoid basidiospores (Ryvarden & Gilbertson 1994, Niemelä et al. 2004). Therefore, we transferred Spongiporus luteocaesius into Cyanosporus as a new combination although without molecular data. For a detailed description, see Oligoporus luteocaesius by Ryvarden & Melo (2014).
Cyanosporus mediterraneocaesius (M. Pieri & B. Rivoire) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819271
Basionym. Postia mediterraneocaesia M. Pieri & B. Rivoire, Bull. Semestriel Féd. Assoc. Mycol. Méditerranéennes 28: 34. 2005.
Specimens examined. France, Bouches du Rhône, St. Rémy de Provence, on Pinus, 11 Nov. 2000, LY BR 1946 (holotype, LY); Bonnieux, 30 Nov. 2011, LY BR 4274 (LY).
Notes — We have examined the type specimen and another specimen from France. Based on morphological characters and phylogenetic analyses, we transferred Postia mediterraneocaesia to Cyanosporus as a new combination. For a detailed description of the species, see Postia mediterraneocaesia by Pieri & Rivoire (2005).
Cyanosporus microporus B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819272; Fig. 3c, 6
Fig. 6.
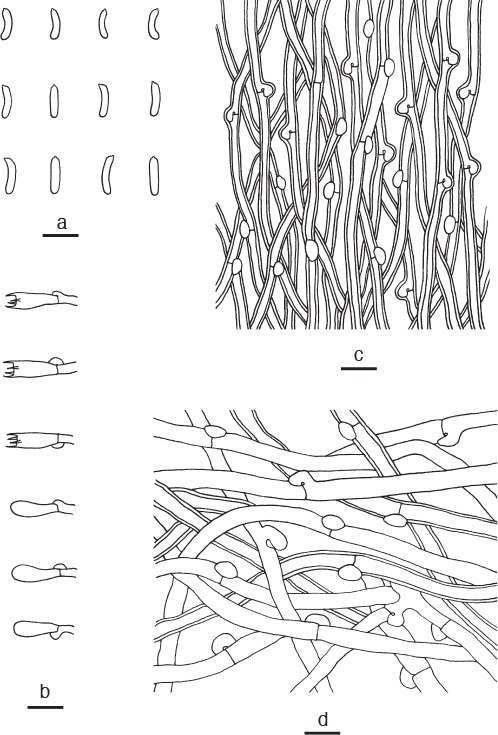
Microscopic structures of Cyanosporus microporus. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Yunnan Province, Pu’er, Taiyanghe National Forest Park, on fallen angiosperm trunk, 8 July 2013, B.K. Cui, Cui 11014 (BJFC 015131).
Etymology. Microporus (Lat.) refers to the small pores.
Diagnosis. Cyanosporus microporus differs from other species in the genus by subrotund pileus, small angular pores, and slightly thick-walled and allantoid basidiospores.
Basidiome annual, pileate, solitary, soft and watery, without odour or taste when fresh, becoming soft corky to fragile upon drying. Pileus subrotund, projecting up to 2.5 cm, 6 cm wide and 1.5 cm thick at base. Pileal surface velutinate, white to cream with blue tint when fresh, becoming smooth, rugose, cream to pinkish buff when dry; margin obtuse, white when fresh, greyish brown when dry. Pore surface white when fresh, bluish when bruised, becoming cream to buff when dry; sterile margin narrow to almost lacking; pores angular, 6–8 per mm; dissepiments thin, entire. Context white to cream, corky, up to 1.3 cm thick. Tubes cream, fragile, up to 2 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 3.5–6 μm diam. Generative hyphae in trama hyaline, thick-walled with a wide lumen, occasionally branched, interwoven, 2–4 μm diam. Cystidia or cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 11–13.5 × 4–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid, hyaline, slightly thick-walled, smooth, IKI–, weakly CB+, (4.2–)4.5–4.9(–5.2) × 1–1.2 μm, L = 4.69 μm, W = 1.08 μm, Q = 4.47–4.52 (n = 60/2).
Additional specimen (paratype) examined. China, Yunnan Province, Chuxiong, Zixishan Nature Reserve, on dead angiosperm tree, 28 Aug. 2010, Y.C. Dai, Dai 11717 (BJFC 008830).
Notes — Cyanosporus alni may be confused with C. microporus by sharing velutinate, white with a blue-grey tint pileal surface and cream to buff pore surface when dry. However, C. alni differs in having bigger pores (5–6 per mm) and relatively longer basidiospores (4.5–6 × 1–1.5 μm; Table 2).
Table 2.
Comparisons of the main morphological characters of species in Amaropostia, Calcipostia, Cyanosporus, Cystidiopostia, Fuscopostia, Oligoporus s.str., Osteina, Postia s.str., Rhodonia and Spongiporus s.str.
| Species | Basidiocarps | Pileal surface when dry | Pores (per mm) | Gloeoplerous hyphae | hyphal pegs | Cystidia | Cystidioles | Basidiospores |
References | |
|---|---|---|---|---|---|---|---|---|---|---|
| L × W (μm) | Q = L/W | |||||||||
| Amaropostia | ||||||||||
| A. hainanensis | P | G | 7–9 | – | – | – | – | 4–5.5 × 1.5–2 | 2.59–2.73 | this study |
| A. stiptica | P | G | 5–6 | – | – | – | + | 3.5–4.5 × 1.5–2 | 2.19–2.38 | this study |
| Calcipostia | ||||||||||
| C. guttulata | P | G | 3–5 | – | – | – | + | 3–4 × 1.8–2.3 | 1.75–1.83 | this study |
| Cyanosporus | ||||||||||
| C. alni | P / ER | V | 5–6 | – | – | – | – | 4.5–6 × 1–1.5 | 4.17–4.35 | this study |
| C. caesius | P / ER | T | 3–6 | + | – | – | – | 4.5–6 × 1.5–2 | 3.18–3.29 | this study |
| C. fusiformis | P / ER | T | 4–5 | – | – | – | + | 4.5–5.2 × 0.8–1.1 | 5.21–5.45 | this study |
| C. luteocaesius | R / ER | T | 3–4 | – | – | – | – | 4.7–6.3 × 1.6–1.9 | 3–3.2 | Niemelä 2005 |
| C. mediterraneocaesius | P / ER | V | 4–5 | – | – | – | – | 5–6 × 1.5–2 | 3.74 | this study |
| C. microporus | P | V | 6–8 | – | – | – | – | 4.5–4.9 × 1–1.2 | 4.47–4.52 | this study |
| C. mongolicus | R / ER | H | 3–4 | – | – | + | + | 4.5–5 × 1.5–1.9 | 2.77–2.85 | this study |
| C. piceicola | P | V | 3–5 | – | – | – | – | 4–4.5 × 0.9–1.3 | 3.75–3.97 | this study |
| C. subcaesius | P / ER | G | 4–5 | – | – | – | – | 4–5 × 1–1.5 | 3.65–3.82 | this study |
| C. subhirsutus | P | H | 2–3 | – | – | – | – | 4–4.5 × 0.9–1.3 | 3.67–3.79 | this study |
| C. tricolor | P | V | 4–5 | – | – | – | – | 4–4.8 × 0.8–1.2 | 4.55–4.87 | this study |
| C. ungulatus | P | G | 4–6 | – | – | – | – | 4.5–5 × 0.9–1.2 | 4.79–4.83 | this study |
| Cystidiopostia | ||||||||||
| C. hibernica | R | – | 2–4 | – | – | + | – | 5–6 × 1–1.5 | 4.58–4.73 | this study |
| C. inocybe | R | – | 3–5 | – | – | + | – | 5–6 × 1.5–1.7 | 3.32–3.59 | this study |
| C. pileata | P / ER | G | 3–4 | – | – | + | – | 3.8–4.8 × 0.9–1.1 | 4.04–4.52 | Dai & Renvall 1994 |
| Fuscopostia | ||||||||||
| F. duplicata | P | G | 3–4 | – | – | + | – | 3.8–5.8 × 1.8–2.5 | 2.28–2.41 | Shen et al. 2014 |
| F. fragilis | P / ER | T | 4–6 | – | – | – | – | 4–6 × 1.7–2.1 | 2.49–2.69 | this study |
| F. lateritia | R / ER | V | 3–4 | – | – | – | + | 4.5–6 × 1.2–1.6 | 3.48–3.76 | this study |
| F. leucomallella | P / ER | G | 3–4 | – | – | + | – | 4.5–6 × 1–1.7 | 3.33–3.65 | this study |
| Oligoporus s.str. | ||||||||||
| O. rennyi | R | – | 2–4 | – | – | – | – | 4.8–6 × 2.5–3.5 | 1.92–2.08 | this study |
| O. sericeomollis | R | – | 4–6 | – | – | + | + | 4–5 × 2–2.5 | 2.05–2.21 | this study |
| Osteina | ||||||||||
| O. obducta | P | G | 3–5 | – | – | – | – | 4–5.2 × 2–2.4 | 2.06–2.2 | Cui et al. 2014 |
| O. undosa | R / ER | G | 2–3 | – | – | – | – | 4.5–6 × 1–1.5 | 4.22–4.38 | this study |
| Postia s.str. | ||||||||||
| P. amurensis | P | G | 3–4 | – | – | + | 4.1–5.2 × 1–1.2 | 3.93–4.18 | Dai & Penttilä 2006 | |
| P. hirsuta | P | H | 3–4 | – | – | – | – | 4–4.8 × 1–1.2 | 4.33–4.35 | Shen & Cui 2014 |
| P. lactea | P | G | 4–5 | + | – | – | – | 4–5 × 1–1.5 | 3.86–4.11 | this study |
| P. lowei | P / ER | V | 3–4 | – | – | – | – | 4.8–5.2 × 1.8–2.2 | 2.52–2.73 | this study |
| P. ochraceoalba | P | G | 6–7 | – | – | – | – | 4–4.5 × 1–1.5 | 3.18–4.02 | Shen et al. 2015 |
| P. sublowei | P / ER | V | 3–4 | – | – | – | + | 4–4.5 × 1–1.5 | 4.48–4.62 | this study |
| P. tephroleuca | P / ER | T | 3–4 | – | – | – | – | 4.5–6 × 1–1.5 | 3.75–3.92 | this study |
| Rhodonia | ||||||||||
| R. obliqua | R | – | 2–3 | + | – | – | – | 4.8–6.3 × 2–2.5 | 2.41–2.53 | Wei & Qin 2010 |
| R. placenta | R | – | 3–4 | + | – | – | – | 5–7 × 2.5–3 | 2.04–2.37 | this study |
| R. rancida | R | – | 2–4 | – | – | – | – | 6–8 × 2–3 | 2.55–2.69 | this study |
| R. subplacenta | R | – | 3–5 | – | – | – | – | 4.2–6 × 1.9–2.4 | 2.37–2.45 | Cui & Li 2012 |
| Spongiporus s.str. | ||||||||||
| S. balsameus | P / ER | G | 5–6 | – | – | + | – | 4–5 × 2.5–3 | 1.86–2.05 | this study |
| S. florifomis | P / ER | G | 6–8 | – | + | – | – | 3.5–4.5 × 2–2.5 | 1.76–1.89 | this study |
| S. gloeoporus | P | V | 3–4 | – | – | – | + | 4–4.5 × 2–2.5 | 1.86–2.16 | Shen et al. 2015 |
| S. leucospongia | P / ER | G | 2–4 | – | + | – | – | 5–8 × 1.2–1.5 | 3.82–4.22 | this study |
| S. zebra | P | G | 7–8 | – | – | – | – | 3.6–4.2 × 2–2.5 | 1.82–1.91 | this study |
Abbreviations used: ER = Effused-reflexed; P = Pileate; R = Resupinate; G = Glabrous; H = Hirsute; T = Tomentose; V = Velutinate; + = Present; – = Absent.
Cyanosporus mongolicus B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819273; Fig. 3d, 7
Fig. 7.
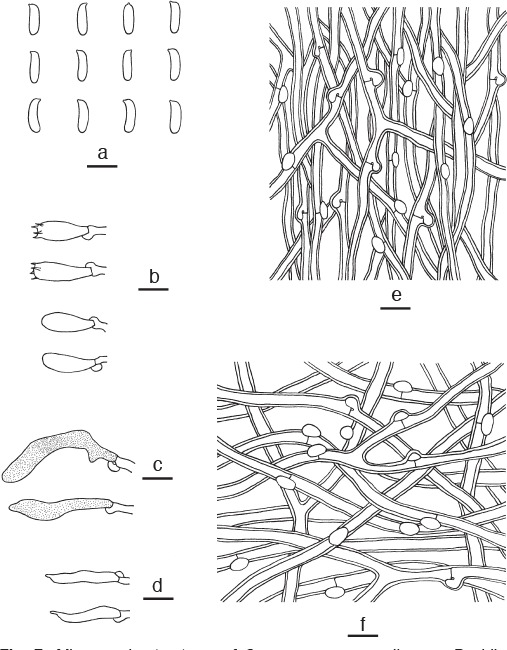
Microscopic structures of Cyanosporus mongolicus. a. Basidiospores; b. basidia and basidioles; c. gloeocystidia; d. cystidioles; e. hyphae from trama; f. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–f = 10 μm.
Holotype. China, Inner Mongolia Autonomous Region, Ewenk, Honghuaerji Nature Reserve, on fallen trunk of Pinus, 19 Oct. 2015, B.K. Cui, Cui 13518 (BJFC).
Etymology. Mongolicus (Lat.) refers to the type locality of Inner Mongolia Autonomous Region in China.
Diagnosis. Cyanosporus mongolicus differs from other species in the genus by resupinate to effused-reflexed basidiocarps, swollen hyphae in KOH, gloeocystidia and cystidioles presenting, and cylindrical to allantoid basidiospores.
Basidiome annual, resupinate to effused-reflexed, solitary, soft and watery, without odour or taste when fresh, becoming soft corky to fragile upon drying. Resupinate part up to 4 cm long, 3 cm wide and 4 mm thick at centre, easily separable from the substrate. Pileus flabelliform, projecting up to 2.5 cm, 4 cm wide and 8 mm thick at base. Pileal surface white to cream when fresh, hirsute, becoming greyish brown; margin acute, white when fresh, fuscous and incurved when dry. Pore surface white to cream when fresh, becoming greyish brown with bluish tint when dry; sterile margin up to 2 mm wide, white when fresh, becoming greyish brown upon drying; pores angular, 3 or 4 per mm; dissepiments thin, entire when juvenile, becoming lacerate with age. Context white, soft corky, up to 5 mm thick. Tubes pale mouse-grey with bluish tint, fragile, up to 3 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; hyphae swollen in KOH. Generative hyphae in context hyaline, thick-walled with a wide lumen, occasionally branched, interwoven, 3.5–5 μm diam. Generative hyphae in trama hyaline, thick-walled with a wide lumen, moderately branched, interwoven, 2–5 μm diam. Gloeocystidia present, shape variable from pyriform to broadly clavate, dark blue in CB, bright yellow in IKI, 20–30 × 5–8 μm; cystidioles present, thin-walled, slim clavate with a narrow apex, 21–25 × 2–3 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 12–14 × 5–7 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores cylindrical to allantoid, hyaline, slightly thick-walled, smooth, IKI–, weakly CB+, (4–)4.5–5(–5.5) × 1.5–1.9(–2) μm, L = 4.94 μm, W = 1.74 μm, Q = 2.77–2.85 (n = 60/2).
Additional specimen (paratype) examined. China, Inner Mongolia Autonomous Region, Ewenk, Honghuaerji Nature Reserve, on fallen trunk of Pinus, 19 Oct. 2015, B.K. Cui, Cui 13519 (BJFC).
Notes — Cyanosporus luteocaesius also produces resupinate basidiocarps and similar sized pores (3 or 4 per mm) as C. mongolicus, but it differs in bright yellow basidiocarps, unchanged hyphae in KOH, absence of cystidioles and longer basidiospores (4.7–6.3 × 1.6–1.9 μm; Niemelä 2005). Cyanosporus caesius resembles C. mongolicus in having white to bluish pore surface, similar sized pores, and similar basidiocarps, but it is distinguished by greyish to bluish pileal surface, presence of gloeoplerous hyphae, and absence of gloeocystidia and cystidioles (Ryvarden & Melo 2014).
Cyanosporus piceicola B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819274; Fig. 3e, 8
Fig. 8.
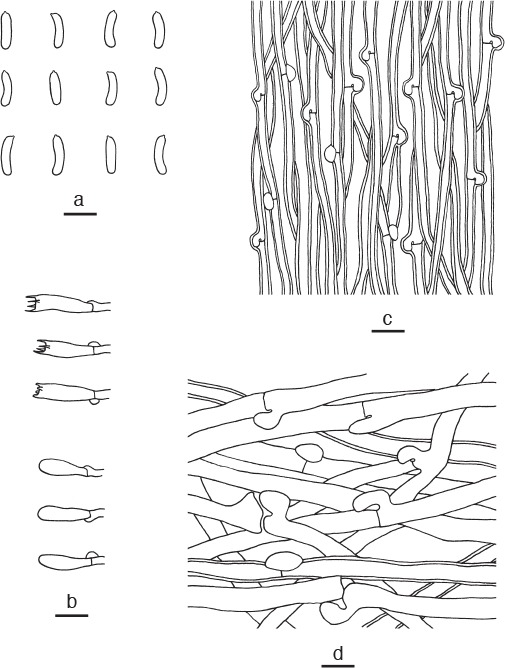
Microscopic structures of Cyanosporus piceicola. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reserve, on stump of Picea, 11 Oct. 2012, B.K. Cui, Cui 10626 (BJFC 013551).
Etymology. Piceicola (Lat.) refers to the species growing on Picea.
Diagnosis. Cyanosporus piceicola differs from other species in the genus by flabelliform pileus, slightly thick-walled and allantoid basidiospores, and specifically growing on Picea.
Basidiome annual, pileate, solitary, soft corky and without odour or taste when fresh, becoming hard corky and light in weight upon drying. Pileus flabelliform, projecting up to 3 cm, 5.5 cm wide and 1.8 cm thick at base. Pileal surface cream to clay-buff, with bluish grey zonation when fresh, velutinate, becoming light greyish brown upon drying; margin acute, concolorous with pileal surface. Pore surface white with bluish tint when fresh, becoming cream when dry; sterile margin up to 1 mm wide, clay-buff when fresh, becoming greyish brown upon drying; pores round, 3–5 per mm; dissepiments thin, entire. Context cream, hard corky, up to 1.5 cm thick. Tubes cream to buff-yellow, hard corky, up to 3 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thin- to slightly thick-walled with a wide lumen, seldom branched, loosely interwoven, 5–7 μm diam. Generative hyphae in trama hyaline, slightly thick-walled with a wide lumen, usually unbranched, parallel along the tubes, 2.5–4 μm diam. Cystidia or cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 13–16 × 4–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid, hyaline, slightly thick-walled, smooth, IKI–, weakly CB+, (3.9–)4–4.5(–4.8) × 0.9–1.3 μm, L = 4.65 μm, W = 1.21 μm, Q = 3.75–3.97 (n = 150/5).
Additional specimens (paratypes) examined. China, Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reserve, on fallen trunk of Picea, 11 Oct. 2012, B.K. Cui, Cui 10617 (BJFC 013542); Xizang Autonomous Region (Tibet), Linzhi County, Sejila Mountain, on fallen trunk of Picea, 18 Sept. 2014, B.K. Cui, Cui 12158 (BJFC 017072); Milin County, Nanyigou Forest Park, on fallen trunk of Picea, 16 Sept. 2014, B.K. Cui, Cui 12088 (BJFC 017002); Yunnan Province, Weixi County, Laojunshan Nature Reserve, on fallen trunk of Picea, 21 Sept. 2011, B.K. Cui, Cui 10446 (BJFC 11341).
Notes — Cyanosporus subcaesius and C. subhirsutus resemble C. piceicola by producing similar basidiospores, but C. subcaesius differs from C. piceicola by glabrous pileal surface, white to pale grey pore surface, and interwoven, thin-walled tramal hyphae (Ryvarden & Melo 2014); while C. subhirsutus is separated by its dish-shaped pileus with hirsute pileal surface, bigger pores (2 or 3 per mm) and interwoven tramal hyphae.
Cyanosporus subcaesius (A. David) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819275
Basionym. Tyromyces subcaesius A. David, Bull. Mens. Soc. Linn. Lyon 43: 120. 1974.
= Oligoporus subcaesius (A. David) Ryvarden & Gilb., Syn. Fungorum 7: 435. 1993.
Specimens examined. Finland, Uusimaa, Helsinki, Arabia, on angiosperm stump, 23 Nov. 1996, Y.C. Dai, Dai 2345 (IFP 015311); Vantaa, on fallen trunk of Prunus, 4 Oct. 1997, Y.C. Dai, Dai 2725 (IFP 015280). – France, Isère, on Malus, Oct.1968, David 652 (holotype, LY); Loire, on Populus, 31 Oct. 2000, LY BR 1868 (LY).
Notes — Cyanosporus subcaesius can be recognized by whitish pileal surface with greyish tints in spots and streaks and pale grey pore surface. It is widespread in Europe. We have examined the type specimen and other specimens from Europe. This species has the typical morphological features of the Cyanosporus caesius group with blue greyish discoloration and similar allantoid basidiospores. Therefore, we proposed it as a new combination of Cyanosporus. For a detailed description of the species, see Oligoporus subcaesius by Ryvarden & Melo (2014).
Cyanosporus subhirsutus B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819276; Fig. 3f, 9
Fig. 9.
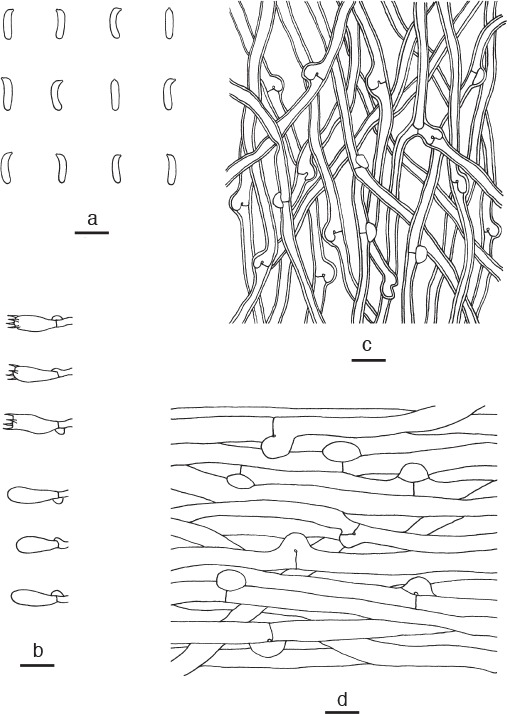
Microscopic structures of Cyanosporus subhirsutus. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Guizhou Province, Jiangkou County, Fanjingshan Nature Reserve, on fallen trunk of Pterocarya, 21 Nov. 2014, Y.C. Dai, Dai 14892 (BJFC 018005).
Etymology. Subhirsutus (Lat.) refers to the morphological similarity to Postia hirsuta.
Diagnosis. Cyanosporus subhirsutus differs from other species in the genus by dish-shaped pileus, hirsute and zonate pileal surface and big pores.
Basidiome annual, pileate, solitary, soft, watery, without odour or taste when fresh, becoming soft corky to fragile upon drying. Pileus dish-shaped, projecting up to 4 cm, 6 cm wide and 0.8 cm thick at base. Pileal surface with pale mouse-grey and cream zones when fresh, becoming cream to buff and hirsute when dry; margin acute, white with a little blue tint when fresh, cream when dry. Pore surface white when fresh, becoming pinkish buff to honey-yellow when dry; sterile margin narrow to almost lacking; pores angular, 2 or 3 per mm; dissepiments thin, entire. Context white, soft corky, up to 5 mm thick. Tubes cream, fragile, up to 3 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thin-walled, rarely branched, regularly arranged, 4–6 μm diam. Generative hyphae in trama hyaline, slightly thick-walled with a wide lumen, occasionally branched, interwoven, 3–4.5 μm diam. Cystidia and cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 10–12 × 4–6 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid, hyaline, slightly thick-walled, smooth, IKI–, weakly CB+, (3.9–)4–4.5 × 0.9–1.3 μm, L = 4.19 μm, W = 1.12 μm, Q = 3.67–3.79 (n = 90/3).
Additional specimens (paratypes) examined. China, Fujian Province, Yongjing County, Huboliao Nature Reserve, on fallen angiosperm branch, 26 Oct. 2013, B.K. Cui, Cui 11330 (BJFC 015446); Yunnan Province, Pu’er, Taiyanghe National Forest Park, on fallen angiosperm trunk, 8 July 2013, B.K. Cui, Cui 11019 (BJFC 015136).
Notes — Postia hirsuta may be confused with Cyanosporus subhirsutus by pale mouse-grey and hirsute pileal surface, yellowish pore surface when dry, and allantoid to cylindrical basidiospores, but P. hirsuta differs in its thick pileus, without bluish margin, and thick-walled contextual hyphae (Shen & Cui 2014).
Cyanosporus tricolor B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819277; Fig. 3g, 10
Fig. 10.
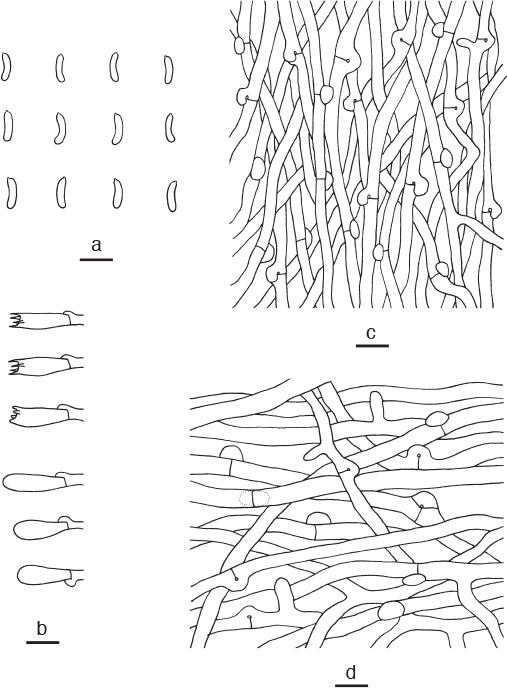
Microscopic structures of Cyanosporus tricolor. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Xizang Autonomous Region (Tibet), Motuo County, on fallen branch of Abies, 20 Sept. 2014, B.K. Cui, Cui 12233 (BJFC 07147).
Etymology. Tricolor (Lat.) refers to white, blue and pale mouse-grey upper surface when fresh.
Diagnosis. Cyanosporus tricolor differs from other species in the genus by semicircular pileus with white, blue and pale mouse-grey upper surface, and vertical projections nearby clamp connections frequently presenting in hyphae.
Basidiome annual, pileate, soft, watery, without odour or taste when fresh, becoming hard corky upon drying. Pileus semicircular, projecting up to 2 cm, 4 cm wide and 1 cm thick at base. Pileal surface light greyish brown with bluish grey zone, velutinate when fresh, becoming greyish brown, glabrous when dry; margin acute, white when fresh, greyish brown when dry. Pore surface white when fresh, becoming cream to buff when dry; sterile margin up to 1 mm wide, white when fresh, clay-buff when dry; pores angular, 4 or 5 per mm; dissepiments thin, entire. Context white, hard corky, up to 9 mm thick. Tubes cream, fragile, up to 1 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thin-walled, occasionally branched, interwoven, 3–5 μm diam, vertical projections nearby clamp connections frequently present. Generative hyphae in trama hyaline, thin-walled, seldom branched, interwoven, 2–3 μm diam, vertical projections occasionally present near to clamp connections. Cystidia and cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 12–15 × 4–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid, hyaline, slightly thick-walled, smooth, IKI–, weakly CB+, (3.9–)4–4.8(–4.9) × 0.8–1.2 μm, L = 4.51 μm, W = 0.97 μm, Q = 4.55–4.87 (n = 90/3).
Additional specimens (paratypes) examined. China, Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Abies, 20 Oct. 2012, B.K. Cui, Cui 10790 (BJFC 013712); on fallen trunk of Picea, 20 Oct. 2012, B.K. Cui, Cui 10780 (BJFC 013702).
Notes — Cyanosporus microporus has similar basidiospores with C. tricolor, but it is easily distinguished from C. tricolor by subrotund pileus, bluish pore surface when bruised and smaller pores (6–8 per mm).
Cyanosporus ungulatus B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819278; Fig. 3h, 11
Fig. 11.
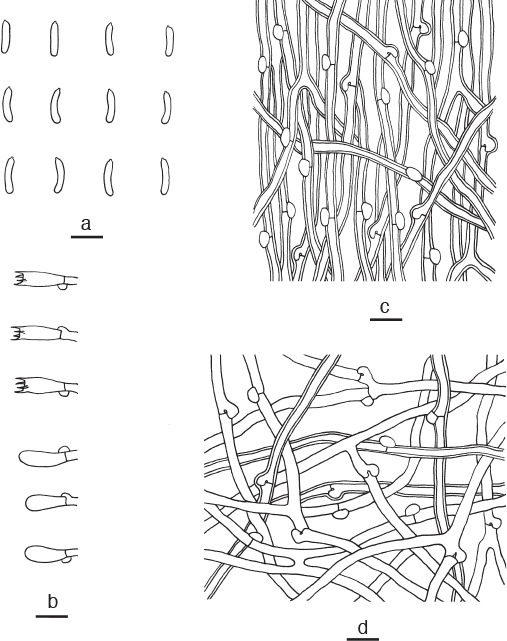
Microscopic structures of Cyanosporus ungulatus. a. Basidiospores; b. basidia and basidioles; c. hyphae from trama; d. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–d = 10 μm.
Holotype. China, Sichuan Province, Mianning County, Lingshan Temple, on fallen branch of Castanopsis, 17 Sept. 2012, Y.C. Dai, Dai 12897 (BJFC 013166).
Etymology. Ungulatus (Lat.) refers to ungulate basidiocarps.
Diagnosis. Cyanosporus ungulatus differs from other species in the genus by ungulate basidiocarps, sulcate pileal surface with olivaceous buff, pinkish buff, cream to ash-grey and white zones when fresh.
Basidiome annual, pileate, ungulate, solitary, soft corky, without odour or taste when fresh, becoming hard and chalky upon drying. Pileus semicircular, projecting up to 1.8 cm, 2 cm wide and 1.5 cm thick at base. Pileal surface sulcate with olivaceous buff, pinkish buff, cream to ash-grey and white zones when fresh, glabrous, slightly darkening when dry; margin acute and white when fresh, cream upon drying. Pore surface white when fresh, becoming cream when dry; sterile margin up to 1 mm wide, concolorous with pore surface; pores round, 4–6 per mm; dissepiments thin, entire. Context cream, hard corky, up to 1.2 cm thick. Tubes cream to buff, hard and chalky, up to 3 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, thin- to slightly thick-walled with a wide lumen, frequently branched, interwoven, 2.5–4.5 μm diam. Generative hyphae in trama hyaline, slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2–3 μm diam. Cystidia and cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 12–15 × 4–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid, hyaline, thin-walled, smooth, IKI–, CB–, 4.5–5(–5.5) × 0.9–1.2 μm, L = 4.86 μm, W = 1.01 μm, Q = 4.79–4.83 (n = 60/2).
Additional specimen (paratype) examined. China, Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Abies, 20 Oct. 2012, B.K. Cui, Cui 10778 (BJFC 013700).
Notes — Phylogenetically, Cyanosporus ungulatus grouped together with C. fusiformis. Both species produce slim thin-walled basidiospores, but C. fusiformis differs from C. ungulatus by its small basidiocarps, darkish pileal surface when dry and the presence of cystidioles.
Cystidiopostia B.K. Cui, L.L. Shen & Y.C. Dai, gen. nov. — MycoBank MB819279
Type species. Cystidiopostia hibernica (Berk. & Broome) B.K. Cui, L.L. Shen & Y.C. Dai.
Etymology. Cystidiopostia (Lat.) refers to the new genus resembling Postia but with apically encrusted cystidia.
Diagnosis. Morphologically, Cystidiopostia differs from Postia s.str. by resupinate basidiocarps and presence of apically encrusted cystidia.
Basidiocarps annual, resupinate to effused-reflexed, or pileate, soft when fresh, fragile when dry. Pileal surface white when fresh, cream to buff when dry, smooth to slightly radially rugose, azonate. Pore surface white when fresh, cream or with yellowish tint upon drying. Context white, soft corky. Tubes white to cream, fragile. Pores round to angular. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Cystidia present, thin- to thick-walled, mostly subulate, usually with a narrow apex. Basidiospores allantoid, hyaline, thin-walled, smooth, IKI–, CB–.
Cystidiopostia hibernica (Berk. & Broome) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819280
Basionym. Polyporus hibernicus Berk. & Broome, Ann. Mag. Nat. Hist., ser. IV, 7: 428. 1871.
= Oligoporus hibernicus (Berk. & Broome) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen branch of Abies, 13 July 2007, Y.C. Dai, Dai 8248 (IFP 005454); Zhejiang Province, Lin’an County, Tianmushan Nature Reserve, on fallen angiosperm trunk, 10 Oct. 2005, B.K. Cui, Cui 2658 (BJFC 002080). – Finland, Kittilän Lappi, Kittila, Jerisjavi, on fallen trunk of Pinus, 30 Aug. 1999, Y.C. Dai, Dai 3189 (IFP 015561); Perä-Pohjanmaa, South Pisavaara National Park, on fallen trunk of Pinus, 15 Sept. 1997, Y.C. Dai, Dai 2653 (IFP 015286). – Ireland, Luggela, on Abies, Sept. 1867, 181070 (holotype, K).
Notes — This species was described from Ireland. We have examined the type specimen and other specimens from China and Europe. Based on morphological characters and phylogenetic analyses, we transferred Oligoporus hibernicus to Cystidiopostia as a new combination. For a detailed description of the species, see Oligoporus hibernicus by Ryvarden & Melo (2014).
Cystidiopostia inocybe (A. David & Malençon) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819281
Basionym. Tyromyces inocybe A. David & Malençon, Bull. Trimestriel Soc. Mycol. France 94: 395. 1978.
= Oligoporus inocybe (A. David & Malençon) Ryvarden & Gilb., Syn. Fungorum 7: 415. 1993.
Specimens examined. China, Heilongjiang Province, Yichun, Fenglin Nature Reserve, on stump of Populus, 8 Sept. 2002, Y.C. Dai, Dai 3706 (IFP 005406). – France, Fleury d’Aude, 28 Nov. 2009, LY BR 3703 (LY).
Notes — This species was originally described from France. Although we did not find the type specimen, we have examined one specimen from France (type locality), and its morphological characters fit well with this species. Based on morphological characters and phylogenetic analyses, we transferred Oligoporus inocybe to Cystidiopostia as a new combination. For a detailed description of the species, see Oligoporus inocybe by Ryvarden & Melo (2014).
Cystidiopostia pileata (Parmasto) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819282
Basionym. Auriporia pileata Parmasto, Mycotaxon 11: 173. 1980.
= Postia pileata (Parmasto) Y.C. Dai & Renvall, Fungal Science 11: 98. 1996.
= Postia amylocystis Y.C. Dai & Renvall, Ann. Bot. Fenn. 31: 72. 1994.
Specimens examined. China, Anhui Province, Huangshan County, Huangshan, on fallen branch of Pinus, 13 Oct. 2004, Y.C. Dai, Dai 6137 (BJFC 002088); Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Abies, 9 Aug. 2011, B.K. Cui, Cui 10034 (BJFC 010927); Liaoning Province, Huanren County, Laotudingzi Nature Reserve, on fallen trunk of Abies, 31 July 2008, B.K. Cui, Cui 5721 (BJFC 003664). – Russia, Far East, Sikhote Alinskij Nature Reserve, 19 Sept. 1967, E. Parmasto (holotype, TAA 52807, isotype in O).
Notes — This species was originally described from the Russia Far East. We have examined the type specimen and other specimens from Northeast China. Based on morphological characters and phylogenetic analyses, we transferred Auriporia pileata to Cystidiopostia as a new combination. For a detailed description of the species, see Auriporia pileata by Núñez & Ryvarden (2001).
Fuscopostia B.K. Cui, L.L. Shen & Y.C. Dai, gen. nov. — MycoBank MB819283
Type species. Fuscopostia fragilis (Fr.) B.K. Cui, L.L. Shen & Y.C. Dai.
Etymology. Fuscopostia (Lat.) refers to the new genus resembling Postia but with brownish basidiocarps when bruised or dried.
Diagnosis. Morphologically, Fuscopostia differs from Postia s.str. by pileal surface and pore surface turning to brownish when bruised.
Basidiocarps annual, resupinate, effused-reflexed or pileate, soft when fresh, fragile when dry. Pileal surface white to cream when fresh, mostly turned to brownish when bruised or dried, tomentose to glabrous, azonate. Pore surface whitish to buff when fresh, soon became reddish to rusty brown when bruised or dried. Context white, corky. Tubes brownish, fragile. Pores round to angular. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Gloeocystidia present or not, cystidioles frequently present. Basidiospores cylindrical to allantoid, hyaline, thin-walled, smooth, IKI–, CB–.
Fuscopostia duplicata (L.L. Shen, B.K. Cui & Y.C. Dai) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819284
Basionym. Postia duplicata L.L. Shen, B.K. Cui & Y.C. Dai, Phytotaxa 162: 149. 2014.
Specimens examined. China, Zhejiang Province, Qingyuan County, Baishanzu Nature Reserve, on rotten angiosperm wood, 14 Aug. 2013, Y.C. Dai, Dai 13411 (holotype, BJFC 014872); Yunnan Province, Lanping County, Tongdian, Luoguqing, on stump of Pinus, 19 Sept. 2011, B.K. Cui, Cui 10366 (paratype, BJFC 011261).
Notes — This species was only found in China. We have examined the type specimen. Based on morphological characters and phylogenetic analyses, we transferred Postia duplicata to Fuscopostia as a new combination. For a detailed description of the species, see Postia duplicata by Shen et al. (2014).
Fuscopostia fragilis (Fr.) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819285
Basionym. Polyporus fragilis Fr., Elench. Fung. 1: 86. 1828.
= Postia fragilis (Fr.) Jülich, Persoonia 11: 423. 1982.
= Oligoporus fragilis (Fr.) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Abies, 9 Aug. 2011, B.K. Cui, Cui 10020 (BJFC 010913); Fusong County, Lushuihe Forest Farm, on fallen trunk of Pinus, 11 Aug. 2011, B.K. Cui, Cui 10088 (BJFC 010981); Yunnan Province, Lanping County, Changyanshan Nature Reserve, on fallen trunk of Picea, 18 Sept. 2011, B.K. Cui, Cui 10306 (BJFC 011201).
Notes — This is a widespread species in temperate areas. The older specimens deposited in the herbaria of BPI, NY and K together with the isotype of its synonym Spongipellis sensibilis have been studied by Lowe (1975). The morphological characters of our studied specimens from China fit well with the description of Lowe (1975). Based on morphological characters and phylogenetic analyses, we transferred Oligoporus fragilis to Fuscopostia as a new combination. For a detailed description of the species, see Oligoporus fragilis by Ryvarden & Melo (2014).
Fuscopostia lateritia (Renvall) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819286
Basionym. Postia lateritia Renvall, Karstenia 32: 44. 1992.
= Oligoporus lateritius (Renvall) Ryvarden & Gilb., Syn. Fungorum 7: 417. 1993.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on rotten wood of Picea, 25 Aug. 2005, Y.C. Dai, Dai 6946 (IFP 011823), 29 Aug. 2005, Y.C. Dai, Dai 7139 (IFP 011844). – Finland, Pohjois-Karjala, Lieksa, Patvinsuo National Park, Autiovaara, on fallen decorticated trunk of Pinus, 3 Oct. 1991, Tuomo Niemelä 5547 (holotype, H); Perä-Pohjanmaa, South Pisavaara National Park, on fallen trunk of Pinus, 15 Sept. 1997, Y.C. Dai, Dai 2662 (BJFC 002083).
Notes — This species was originally described from Finland. We have examined the type specimen and other specimens from Finland and China. Based on morphological characters and phylogenetic analyses, we transferred Postia lateritia to Fuscopostia as a new combination. For a detailed description of the species, see Postia lateritia by Renvall (1992).
Fuscopostia leucomallella (Murrill) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819287
Basionym. Tyromyces leucomallellus Murrill, Bull. Torrey Bot. Club 67: 63. 1940.
= Oligoporus leucomallellus (Murrill) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reserve, on fallen trunk of Abies, 11 Oct. 2012, B.K. Cui, Cui 10593 (BJFC 013518); Xizang Autonomous Region (Tibet), Bomi County, on fallen trunk of Pinus, 20 Sept. 2010, B.K. Cui, Cui 9577 (BJFC 008515), Cui 9599 (BJFC 008537); Linzhi County, Kadinggou Forest Park, on fallen trunk of Abies, 24 Sept. 2014, B.K. Cui, Cui 12320 (BJFC 017234). – Finland, Etelä-Häme, Sudenpesänkangas old Forest, on rotten wood of Pinus, 19 Sept. 1996, Y.C. Dai, Dai 2291 (BJFC 002085).
Notes — This is a widespread species in temperate areas of Europe, North America and East Asia. The older specimens, including the paratypes deposited in the herbaria of BPI, FLAS, PC, PR and S, have been studied by Lowe (1975). The morphological characters of our studied specimens from China and Finland fit well with the descriptions of Lowe (1975) and Ryvarden & Melo (2014). Based on morphological characters and phylogenetic analyses, we transferred Oligoporus leucomallellus to Fuscopostia as a new combination. For a detailed description of the species, see Oligoporus leucomallellus by Ryvarden & Melo (2014).
Oligoporus Bref., Unters. Gesammtgeb. Mykol. 8: 114. 1888 — MycoBank MB18144
Type species. Oligoporus rennyi (Berk. & Broome) Donk.
Basidiocarps annual, resupinate, easily separable, soft, gossypine when fresh, soft corky when dry. Pore surface white to cream when fresh, becoming yellowish to pale brown upon drying; margin narrow, whitish, tomentose. Context white, very thin, soft corky. Tubes white, corky when dry. Pores angular. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Cystidia present or not. Basidiospores oblong ellipsoid to ellipsoid, hyaline, slightly thick-walled, smooth, IKI–, CB+; chlamydospores occasionally present, subglobose to ellipsoid, thick-walled, strongly CB+.
Specimens examined. Oligoporus rennyi. China, Heilongjiang Province, Hegang, Lianying Forest Farm, on fallen trunk of Pinus, 30 Aug. 2008, H.S. Yuan, Yuan 5194 (IFP 014196). Oligoporus sericeomollis. Belgium, Louvain, Louvain-la-Neuve, on fallen trunk of Larix, 3 July 2005, Y.C. Dai, Dai 7458 (BJFC 001308). – China, Xizang Autonomous Region (Tibet), Bomi County, on fallen trunk of Pinus, 20 Sept. 2010, B.K. Cui, Cui 9560 (BJFC 008498); Heilongjiang Province, Yichun, Fenglin Nature Reserve, on rotten wood of Picea, 2 Aug. 2011, B.K. Cui, Cui 9870 (BJFC 010763). – Finland, Sompion Lappi, Sodankylä, on charred wood of Pinus, 5 Aug. 1998, Y.C. Dai, Dai 2776 (BJFC 001309). – Norway, Oslo, Botanical Garden, on rotten wood of Picea, 9 Nov. 2011, Y.C. Dai, Dai 12675 (BJFC 012258).
Notes — The name Oligoporus was usually treated as a synonym of Postia. Some mycologists supported the use of Oligoporus (Gilbertson & Ryvarden 1987, Ryvarden & Gilbertson 1994, Núñez & Ryvarden 2001, Bernicchia 2005), while other mycologists preferred to use Postia instead (Renvall 1992, Niemelä et al. 2005, Wei & Dai 2006, Hattori et al. 2011, Cui & Li 2012, Pildain & Rajchenberg 2013). In our study, we propose the use of Postia s.str. for taxa with thin-walled basidiospores, Oligoporus s.str. for taxa having thick-walled and cyanophilous basidiospores (see also Erkkilä & Niemelä (1986) and Renvall (1992)). Phylogenetically, O. rennyi and O. sericeomollis form a well-supported monophyletic lineage (Fig. 1, 2), which is distant from Postia s.str.
Osteina Donk, Schweiz. Z. Pilzk. 44: 86. 1966 — MycoBank MB18164
Type species. Osteina obducta (Berk.) Donk.
Basidiocarps annual, effused-reflexed to pileate or stipitate, watery to fleshy, without odour or taste when fresh, become bone hard when dry; pileal margin characteristically undulate. Pileal surface white when fresh, cream to greyish brown after drying. Pore surface white to cream when fresh, becoming yellowish to yellowish brown when dry; pores angular to irregular. Context white, fleshy when fresh, becoming hard corky when dry. Tubes pale white to yellow, fleshy when fresh, cream to yellowish brown, brittle when dry. Hyphal system monomitic; generative hyphae with clamp connections, thick-walled, IKI–, CB–. Cystidia or cystidioles absent. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–.
Specimens examined. Osteina obducta. China, Heilongjiang Province, Yichun, Fenglin Nature Reserve, on fallen trunk of Betula, 1 Aug. 2011, B.K. Cui, Cui 9832 (BJFC 010725); on fallen trunk of Pinus, 1 Aug. 2011, B.K. Cui, Cui 9825 (BJFC 010718); on fallen trunk of Picea, 2 Aug. 2011, B.K. Cui, Cui 9865 (BJFC 010758); Tahe County, Huzhong Nature Reserve, on root of Larix, 18 Aug. 2003, Y.C. Dai, Dai 4756 (IFP 003369), Dai 4796 (IFP 003370); Inner Mongolia Autonomous Region, Arshan County, Arshan Nature Reserve, on rotten wood of Larix, 31 July 2005, B.K. Cui, Cui 2017 (IFP 003341); Genhe County, Great Hinggan Nature Reserve, on rotten wood of Larix, 27 Aug. 2009, Y.C. Dai, Dai 11024 (IFP 008504); Jilin Province, Antu County, Changbaishan Nature Reserve, on root of Larix, 10 Aug. 1997, Y.C. Dai, Dai 2360 (IFP 003340); 13 Sept. 2007, Y.C. Dai, Dai 9519 (IFP 003348); 1 Aug. 2008, Y.C. Dai, Dai 10076 (IFP 008243); on fallen trunk of Abies, 10 Aug. 2011, B.K. Cui, Cui 10074 (BJFC 010967); on living tree of Pinus, 8 Aug. 2011, B.K. Cui, Cui 9959 (BJFC 010852), B.K. Cui, Cui 9957 (BJFC 010850); Xinjiang Autonomous Region, Buerjin County, Kanasi Nature Reserve, on rotten Larix, 12 Aug. 2004, Y.L. Wei, Wei 1444 (IFP 003382). – Czech Republic, Moravia, Chroustov, on Pinus, 22 July 1996, Laznicka (H); Obora, Hluboká, Velký Kameník, on Larix, Aug. 2002, J. Vlasák 0208/8 (JV). – Russia, Khabarovsk Reg., Solnechny Dist., Suluk-Makit, on Larix, 20 Aug. 2011, Spirin 4238 (H). – USA, Pennsylvania, Ricketts Glen Sate Park, Wilkes-Barre, on Tsuga, July 2003, J. Vlasák 0307/6-J (JV); Washington, Olympic Peninsula, Soleduck River, gymnosperm wood, 3 July 1957, Lowe 7954 (H).
Notes — Osteina was introduced by Donk (1966), but the genus has not been widely accepted and was treated as a synonym of Oligoporus. Cui et al. (2014) used ITS rDNA sequences to infer the phylogenetic position of Osteina in Fomitopsidaceae and defined Osteina obducta as the valid name of the species rather than Oligoporus obductus. In our phylogenetic analyses, the species of Osteina form a single lineage with high support (Fig. 1, 2), and is distinct from Postia s.str. Morphologically, Osteina differs from Postia s.str. by its bone hard basidiocarps when dried, characteristically undulate margin and lacerate pores in older fruitbodies.
Osteina undosa (Peck) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819288
Basionym. Polyporus undosus Peck, Ann. Rep. N.Y. State Mus. Nat. Hist. 34: 42. 1881.
= Postia undosa (Peck) Jülich, Persoonia 11: 424. 1982.
= Oligoporus undosus (Peck) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on rotten wood of Picea, 25 Aug. 2005, Y.C. Dai, Dai 6942 (IFP 011822); 28 Aug. 2005, Y.C. Dai, Dai 7105 (IFP 011838); Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reserve, on fallen trunk of Picea, 12 Oct. 2002, Y.C. Dai, Dai 4062 (IFP 005517). – Finland, Etelä-Häme, Lammi Biological Station, on fallen trunk of Picea, 9 Oct. 1992, Y.C. Dai, Dai 209 (IFP 015316); Sudenpesänkangas Old Forest, on fallen trunk of Picea, 19 Sept. 1996, Y.C. Dai, Dai 2292 (IFP 015317).
Notes — This species is widely distributed in North America, Europe and East Asia. The older specimens deposited at the herbaria of BPI, FH, NY, NYS and SYRF have been extensively studied by Lowe (1975). Although we did not find the type specimen, we have examined the specimens from China and Finland. The morphological characters of our studied specimens fit well with the description of Lowe (1975) and Ryvarden & Melo (2014). Based on morphological characters and phylogenetic analyses, we transferred Oligoporus undosus to Osteina as a new combination. For a detailed description of the species, see Oligoporus undosus by Ryvarden & Melo (2014).
Postia Fr., Hymenomyc. Eur.: 586. 1874 — MycoBank MB18356
Type species. Postia lactea (Fr.) P. Karst.
Basidiocarps annual, effuse-reflexed to pileate, corky when dry. Pileal surface white or greyish to pale greyish brown, smooth or velutinate to hirsute when fresh, cream to greyish brown with some streaks or lines when dry. Pore surface white when fresh, cream to buff or pale reddish brown when dry. Context cream, corky. Tubes white to cream, corky to fragile. Hyphal system monomitic, generative hyphae with clamp connections, IKI–, CB–. Basidiospores allantoid to cylindrical, hyaline, thin-walled, smooth, IKI–, CB–.
Specimens examined. Postia amurensis. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Alnus, 1 Sept. 1993, Y.C. Dai, Dai 903 (holotype, IFP 015745); Liaoning Province, Kuandian County, Baishilazi Nature Reserve, on fallen trunk of Acer, 31 Aug. 2004, B.K. Cui, Cui 1044 (BJFC 013486). Postia calcarea. China, Anhui Province, Huangshan County, Yellow Mts National Park, on fallen angiosperm trunk, 13 Oct. 2004, Y.C. Dai, Dai 6167 (holotype, IFP), Y.C. Dai, Dai 6185 (paratype, IFP); Zhejiang Province, Lin’an County, Tianmushan Nature Reserve, 14 Oct. 2004, Y.C. Dai, Dai 6301 (paratype, IFP). Postia cana. China, Shanxi Province, Qinshui County, Lishan Nature Reserve, on dead fallen trunk of Picea, 15 Sept. 2006, H.S. Yuan, Yuan 2443 (holotype, IFP); on fallen trunk of Picea, 15 Sept. 2006, H.S. Yuan, Yuan 2417 (paratype, IFP); on stump of Picea, 15 Sept. 2006, H.S. Yuan, Yuan 2429 (paratype, IFP), H.S. Yuan, Yuan 2452 (paratype, IFP). Postia gloeocystidia. China, Zhejiang Province, Lin’an County, Tianmushan Nature Reserve, on Pinus, 14 Oct. 2004, Y.C. Dai, Dai 6338 (holotype, IFP), Y.C. Dai, Dai 6327 (paratype, IFP). Postia hirsuta. China, Shaanxi Province, Zhashui County, Niubeiliang Forest Park, on fallen angiosperm trunk, 16 Sept. 2013, B.K. Cui, Cui 11237 (holotype, BJFC 015352); Taibai Mountains, Honghegu Forest Park, on fallen angiosperm trunk, 10 Sept. 2013, B.K. Cui, Cui 11180 (paratype, BJFC 015295). Postia lactea. China, Heilongjiang Province, Tangyuan County, Daliangzihe National Forest Park, on fallen trunk of Pinus, 25 Aug. 2014, B.K. Cui, Cui 11511 (BJFC 016753); Shandong Province, Mengyin County, Mengshan Forest Park, on fallen trunk of Pinus, 17 Aug. 2009, B.K. Cui, Cui 7156 (BJFC 005643), Cui 7167 (BJFC 005654); Xinjiang Autonomous Region, Gongliu County, Xitianshan Nature Reserve, on fallen trunk of Populus, 14 Sept. 2015, Y.C. Dai, Dai 15946 (BJFC 020047); Xizang Autonomous Region (Tibet), Linzhi County, Sejila Mountain, on fallen trunk of Abies, 17 Sept. 2010, B.K. Cui, Cui 9319 (BJFC 008258); on fallen trunk of Picea, 17 Sept. 2014, B.K. Cui, Cui 12141 (BJFC 017055). – Finland, Etelä-Häme, North Kotinen Forest, on fallen trunk of Pinus, 12 Sept. 1997, Y.C. Dai, Dai 2627 (BJFC 002081); Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Betula, 4 Nov. 2011, Y.C. Dai, Dai 12643 (BJFC 012225). Postia lowei. China, Jilin Province, Changbaishan Nature Reserve, on fallen trunk of Pinus, 30 July 1993, Y.C. Dai, Dai 865 (BJFC 013412); Xizang Autonomous Region (Tibet), Bomi County, on fallen trunk of Pinus, 20 Sept. 2010, B.K. Cui, Cui 9585 (BJFC 008523). Postia ochraceoalba. China, Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Picea, 20 Oct. 2012, B.K. Cui, Cui 10802 (holotype, BJFC 013724), B.K. Cui, Cui 10825 (paratype, BJFC 13747); Xizang Autonomous Region (Tibet), Linzhi County, Kadinggou Forest Park, on stump of Abies, 24 Sept. 2014, B.K. Cui, Cui 12333 (BJFC 017247). Postia qinensis. China, Shaanxi Province, Huayin County, Huashan Park, on rotten wood of Pinus tabuliformis, 6 Aug. 2006, Y.C. Dai, Dai 7723 (holotype, IFP). Postia subundosa. China, Heilongjiang Province, Yichun, Fenglin Nature Reserve, on Picea, 7 Sept. 2002, Y.C. Dai, Dai 3608 (holotype, IFP), Y.C. Dai, Dai 3633 (paratype, IFP), Y.C. Dai, Dai 3628 (paratype, IFP). Postia tephroleuca. China, Jiangxi Province, Jiujiang, Lushan Mountain, on fallen trunk of Abies, 9 Oct. 2008, B.K. Cui, Cui 6020 (BJFC 003876); Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Picea, 9 Aug. 2011, B.K. Cui, Cui 10047 (BJFC 010940). – Finland, Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Betula, 3 Nov. 2011, Y.C. Dai, Dai 12603 (BJFC 012225), Y.C. Dai, Dai 12610 (BJFC 012196).
Notes — In our 7-gene based phylogenetic study (Fig. 2), Postia amurensis, P. hirsuta, P. lactea, P. lowei, P. ochraceoalba, P. tephroleuca, and a new species from China form a monophyletic lineage with high support (100% MP, 98% BS, 0.99 BPP). These seven species share similar morphological characters and form the core group of Postia.
Postia sublowei B.K. Cui, L.L. Shen & Y.C. Dai, sp. nov. — MycoBank MB819289; Fig. 3i, 12
Fig. 12.
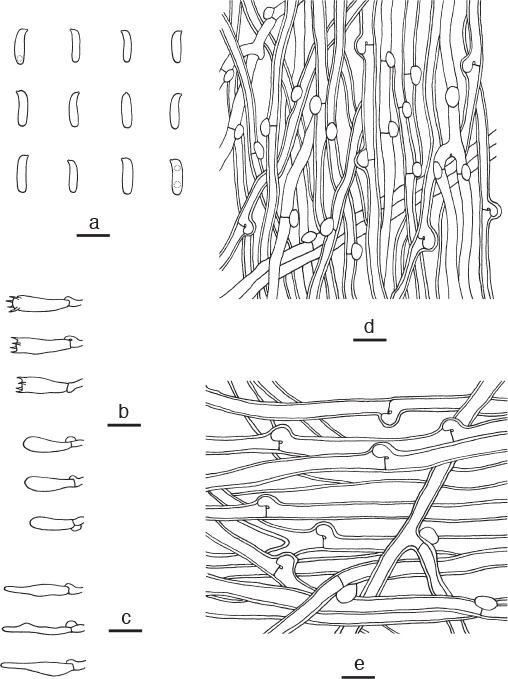
Microscopic structures of Postia sublowei. a. Basidiospores; b. basidia and basidioles; c. cystidioles; d. hyphae from trama; e. hyphae from context (all: holotype). — Scale bars: a = 5 μm; b–e = 10 μm.
Holotype. China, Xizang Autonomous Region (Tibet), Bomi County, on fallen trunk of Picea, 20 Sept. 2010, B.K. Cui, Cui 9597 (BJFC 008535).
Etymology. Sublowei (Lat.) refers to the morphological similarity to Postia lowei.
Diagnosis. Postia sublowei differs from other species in the genus by white pileal surface with pale orange tint when fresh, and fusoid cystidioles presenting in hymenium.
Basidiome annual, pileate or effused-reflected, solitary or in small clusters, soft corky, without odour or taste when fresh, brittle and light in weight when dry. Pileus semicircular, projecting up to 1 cm, 2 cm wide and 0.5 cm thick at base. Pileal surface basically white, with a pale orange tint when fresh, velutinate, becoming cream to greyish brown, glabrous; margin obtuse, white when fresh, fuscous and incurved when dry. Pore surface white when fresh, becoming cream to buff when dry; sterile margin narrow to almost lacking, white when fresh, becoming greyish brown upon drying; pores angular, 3 or 4 per mm; dissepiments thin, entire. Context white, corky, up to 1 mm thick. Tubes cream, fragile to brittle, up to 4 mm long. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH. Generative hyphae in context hyaline, slightly thick-walled with a wide lumen, moderately branched, loosely interwoven, 4–6.5 μm diam. Generative hyphae in trama hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, more or less parallel along the tubes, 3–4.5 μm diam. Cystidia absent; cystidioles present, fusoid, hyaline, thin-walled, 17–20 × 2–4 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 16–20 × 4–4.5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores allantoid to cylindrical, hyaline, thin-walled, smooth, occasionally bearing one or two guttules, IKI–, CB–, 4–4.5(–5) × 1–1.5 μm, L = 4.78 μm, W = 1.06 μm, Q = 4.48–4.62 (n = 60/2).
Additional specimen (paratype) examined. China, Xizang Autonomous Region (Tibet), Bomi County, on fallen trunk of Picea, 20 Sept. 2010, B.K. Cui, Cui 9601 (BJFC 008539).
Notes — Postia lowei resembles P. sublowei by having brittle basidiocarps with greyish brown pileal surface when dry, similar sized pores, but P. lowei differs in whitish pileal surface without pale orange tint when fresh, the absence of cystidioles and wider basidiospores (4.8–5.2 × 1.8–2.2 μm; Table 2).
Rhodonia Niemelä, Karstenia 45: 79. 2005 — MycoBank MB500978
Type species. Rhodonia placenta (Fr.) Niemelä, K.H. Larss. & Schigel.
Basidiocarps annual, resupinate, fairly thick, soft and watery when fresh, corky to brittle when dry. Pore surface white to cream or pale rose-coloured when fresh, becoming cream to buff or brownish when dry; pores round to angular. Context white to red-brown, very thin, corky. Tubes cream to reddish brown, brittle when dry. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Gloeoplerous hyphae occasionally present. Cystidia or cystidioles absent. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–.
Specimens examined. Rhodonia placenta. China, Xinjiang Autonomous Region, Buerjin County, Kanasi Nature Reserve, on stump of Larix, 12 Aug. 2004, Y.L. Wei, Wei 1406 (BJFC 002092). – Finland, Uusimaa, Helsinki, Botanical Garden, 28 Aug. 2007, on dead tree of Salix, Y.C. Dai, Dai 8288 (IFP 015301); Pohjois Karjala, Patvinsuo National Park, on fallen trunk of Pinus, 2 Aug. 1995, Y.C. Dai, Dai 1949 (BJFC 002093). – Russia, Bashkortostan, Uchaly Dist., on fallen trunk of Pinus, 25 Aug. 2001, Y.C. Dai, Dai 11143 (IFP 015300).
Notes — In our study, Rhodonia placenta is clustered with three other species and form a well-supported lineage (Fig. 1, 2) in the antrodia clade. Morphologically, Rhodonia differs from Postia s.str. by its fairly large, thick resupinate basidiocarps and big cylindrical basidiospores.
Rhodonia obliqua (Y.L. Wei & W.M. Qin) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819290
Basionym. Postia obliqua Y.L. Wei & W.M. Qin, Sydowia 62: 166. 2010.
Specimens examined. China, Xizang Autonomous Region (Tibet), Linzhi County, Sejila Mountain, on stump of Larix, 4 Aug. 2004, Y.C. Dai, Dai 5724 (holotype, IFP 015757), Y.C. Dai, Dai 5728 (paratype, IFP 015758), Y.C. Dai, Dai 5730 (paratype, IFP 015759); Yunnan Province, Weixi County, Laojunshan Nature Reserve, on fallen trunk of Picea, 21 Sept. 2011, B.K. Cui, Cui 10470 (BJFC 011365).
Notes — This species was only found in China. We have examined the type specimen. Based on morphological characters and phylogenetic analyses, we transferred Postia obliqua to Rhodonia as a new combination. For a detailed description of the species, see Postia obliqua by Wei & Qin (2010).
Rhodonia rancida (Bres.) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819291
Basionym. Poria rancida Bres., Fungi Trident. 2: 96. 1900.
= Oligoporus rancidus (Bres.) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. Austria, Trient, Tirol, on Larix, 1896, Bresadola, Overholts 25368 (holotype, PACMA 000994). – China, Xizang Autonomous Region (Tibet), Linzhi County, Kadinggou Forest Park, on stump of Pinus, 24 Sept. 2014, B.K. Cui, Cui 12317 (BJFC 017231), Cui 12339 (BJFC 017253).
Notes — We have examined the type specimen and other specimens of this species. Based on morphological characters and phylogenetic analyses, we transferred Oligoporus rancidus to Rhodonia as a new combination. For a detailed description of the species, see Oligoporus rancidus by Ryvarden & Melo (2014).
Rhodonia subplacenta (B.K. Cui) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819292
Basionym. Postia subplacenta B.K. Cui, Mycotaxon 120: 232. 2012.
Specimens examined. China, Heilongjiang Province, Ning’an County, Jingbohu Forest Park, 5 Sept. 2013, on stump of Picea, Y.C. Dai, Dai 13456 (BJFC 014917); Yichun, Fenglin Nature Reserve, on stump of Pinus, 1 Aug. 2011, B.K. Cui, Cui 9818 (paratype, BJFC 010711); Jilin Province, Antu County, Changbaishan Nature Reserve, on fallen trunk of Pinus, 8 Aug. 2011, B.K. Cui, Cui 10001 (holotype, BJFC 010894).
Notes — This species was only found in China. We have examined the type specimen. Based on morphological characters and phylogenetic analyses, we transferred Postia subplacenta to Rhodonia as a new combination. For a detailed description of the species, see Postia subplacenta by Cui & Li (2012).
Spongiporus Murrill, Bull. Torrey Bot. Club 32: 474. 1905 — MycoBank MB18577
Type species. Spongiporus leucospongia (Cooke & Harkn.) Murrill.
Basidiocarps annual, pileate or effused-reflexed, pilei usually imbricate, soft to fibrous when fresh, without odour or taste, corky and slightly fragile upon drying. Pileal surface white when fresh, turning to buff to brownish when dry, velutinate or glabrous, azonate or zonate. Pore surface whitish to buff when fresh, colour unchanged when dry. Context white, corky. Tubes brownish, fragile. Pores round to angular. Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–. Hyphal pegs occasionally present. Cystidia present or not. Basidiospores cylindrical to allantoid, hyaline, thin-walled, smooth, IKI–, CB–.
Specimens examined. Spongiporus balsameus. China, Heilongjiang Province, Yichun, Fenglin Nature Reserve, on angiosperm stump, 1 Aug. 2011, B.K. Cui, Cui 9835 (BJFC 010728); Yunnan Province, Baoshan, Gaoligong Nature Reserve, on fallen angiosperm trunk, 26 Oct. 2009, B.K. Cui, Cui 8207 (BJFC 006696). – Czech Republic, Hluboka, on stump of Picea, 20 Nov. 2011, Y.C. Dai, Dai 12691 (BJFC 012275). Spongiporus leucospongia. USA, California, J.B.Ellis 3731 (K); Pinnacles, Crater Lake National Park, J. Vlasák 0709/123-J (JV).
Notes — Spongiporus, typified by S. leucospongia, was originally established by Murrill (1905), and then it was treated as a genus for all brown-rot species with a monomitic hyphal system (David 1980). However, it had been always regarded as a synonym of Oligoporus or Postia (Pildain & Rajchenberg 2013, Ryvarden & Melo 2014). Since molecular techniques are widely used in taxonomy, the genus is restricted to species with the above definition. In our phylogenetic study (Fig. 1, 2), five species of Spongiporus form a separated lineage in the antrodia clade, they share similar morphological characters and form the core group of Spongiporus s.str.
Spongiporus florifomis (Quél.) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819293
Basionym. Polyporus floriformis Quél., Fungi Trident. 1: 61. 1884.
= Postia floriformis (Quél.) Jülich, Persoonia 11: 423. 1982.
= Oligoporus floriformis (Quél.) Gilb. & Ryvarden, Mycotaxon 22: 365. 1985.
Specimens examined. China, Yunnan Province, Kunming, Heilongtan Park, on gymnosperm stump, 25 July 2014, Y.C. Dai, Dai 13887 (BJFC 017617); Laping County, Changyanshan Nature Reserve, on fallen angiosperm trunk, 18 Sept. 2011, B.K. Cui, Cui 10292 (BJFC 011187); on fallen trunk of Pinus, 19 Sept. 2011, B.K. Cui, Cui 10401 (BJFC 011296). – Spain, Cadiz Province, Sierra Grazalema Natural Park, on fallen trunk of Abies, 22 Nov. 2005, Y.C. Dai, Dai 7441 (BJFC 001300).
Notes — This species is widely distributed in North America, Europe and East Asia. The isotype deposited at BPI has been studied by Lowe (1975). We have examined the specimens from China and Europe. The morphological characters of our studied specimens fit well with the descriptions of Lowe (1975) and Ryvarden & Melo (2014). Based on morphological characters and phylogenetic analyses, we transferred Oligoporus floriformis to Spongiporus as a new combination. For a detailed description of the species, see Oligoporus floriformis by Ryvarden & Melo (2014).
Spongiporus gloeoporus (L.L. Shen, B.K. Cui & Y.C. Dai) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819294
Basionym. Postia gloeopora L.L. Shen, B.K. Cui & Y.C. Dai, Mycol. Progr. 14: 7. 2015.
Specimens examined. China, Xizang Autonomous Region (Tibet), Bomi County, on stump of Pinus, 19 Sept. 2010, B.K. Cui, Cui 9507 (holotype, BJFC 008445), 20 Sept. 2010, B.K. Cui, Cui 9517 (paratype, BJFC 008455).
Notes — This species was only found in China. We have examined the type specimen. Based on morphological characters and phylogenetic analyses, we transferred Postia gloeopora to Spongiporus as a new combination. For a detailed description of the species, see Postia gloeopora by Shen et al. (2015).
Spongiporus zebra (Y.L. Wei & W.M. Qin) B.K. Cui, L.L. Shen & Y.C. Dai, comb. nov. — MycoBank MB819295
Basionym. Postia zebra Y.L. Wei & W.M. Qin, Sydowia 62: 167. 2010.
Specimens examined. China, Jilin Province, Antu County, Changbaishan Nature Reserve, on rotten stump of Abies, 29 Aug. 2005, Y.C. Dai, Dai 7131 (holotype, IFP 015764); 8 Aug. 2011, B.K. Cui, Cui 9973 (BJFC 010866).
Notes — This species was only found in China. We have examined the type specimen. Based on morphological characters and phylogenetic analyses, we transferred Postia zebra to Spongiporus as a new combination. For a detailed description of the species, see Postia zebra by Wei & Qin (2010).
DISCUSSION
Recently, many studies focused on the taxonomy and phylogeny of different brown-rot fungal groups in the antrodia clade (Binder et al. 2005, 2013, Lindner & Banik 2008, Rajchenberg et al. 2011, Cui & Dai 2013, Ortiz-Santana et al. 2013, Pildain & Rajchenberg 2013, Spirin et al. 2013a, b, 2015, 2016a, b, Cui et al. 2014, Song et al. 2014, Shen et al. 2014, 2015, Chen & Cui 2016, Han et al. 2016, Spirin 2016, Chen et al. 2017, Justo et al. 2017, Song & Cui 2017), and species of Postia s.lat. were included in different subclades in the antrodia clade (Binder et al. 2005, Ortiz-Santana et al. 2013, Pildain & Rajchenberg 2013, Cui et al. 2014, Justo et al. 2017). Our phylogenetic results were consistent with previous studies on polyphyly nature of Postia s.lat.
The phylogenies inferred from the combined datasets of 3-gene sequences (Fig. 1) and 7-gene sequences (Fig. 2) strongly support the segregation of Amaropostia, Calcipostia, Cyanosporus, Cystidiopostia, Fuscopostia, Spongiporus from Postia s.str. Morphologically, Cyanosporus, Fuscopostia, Spongiporus, all have pileate or effused-reflexed basidiocarps with corky to slightly fragile pileus when dry and mild taste, but Cyanosporus differs from Fuscopostia and Spongiporus by its more or less bluish basidiocarps and weakly cyanophilous basidiospores; Fuscopostia differs from Spongiporus by pileal surface and pore surface turning brownish when bruised. Amaropostia and Calcipostia share pileate basidiocarps with bitter taste, but Calcipostia differs from Amaropostia by its large and calcareous basidiocarps with circular guttulate depressions on pileal surface.
Morphologically, Oligoporus s.str. is different from Postia s.str. by resupinate, gossypine basidiocarps and thick-walled, cyanophilous basidiospores; moreover, species in Oligoporus s.str. mostly grow on gymnosperm wood, while species in Postia s.str. were reported on both angiosperm and gymnosperm wood (Donk 1971, Ryvarden & Melo 2014). In the current study, species of Oligoporus s.str. form a monophyletic lineage, distant from Postia s.str. (Fig. 1, 2).
Rhodonia was established by Niemelä et al. (2005) based on previous phylogenetic studies (Boidin et al. 1998, Kim et al. 2001, Binder et al. 2005), in which R. placenta was distinct from the bulk of species in Postia. The genus is characterized by annual, resupinate, fairly thick, soft and watery basidiocarps, white or pale rose-coloured pore surface, a monomitic hyphal system with clamped generative hyphae, and thin-walled, smooth, cylindrical basidiospores (Cui et al. 2014). These morphological characters are applicable for P. obliqua, P. rancida and P. subplacenta, and the transfer of these three species to Rhodonia is strongly supported by the phylogenetic analyses (Fig. 1, 2).
Compared with previous studies (Binder et al. 2005, Ortiz-Santana et al. 2013, Pildain & Rajchenberg 2013), we used more samples and more gene markers to make an extensive understanding of the phylogenetic relationships within species in Postia and related genera. Our current phylogeny (Fig. 1) inferred from the combined 3-gene sequences demonstrated 12 major lineages for the 45 sampled species of Postia s.lat. However, four Postia species from Argentina, namely P. pelliculosa, P. punctata, P. dissecta and P. carbophila, weakly grouped with species in Postia s.str., of which P. pelliculosa and P. punctata consistently formed a separated lineage with high support (100 % MP, 100 % BS, 1.00 BPP) in accordance with Pildain & Rajchenberg (2013), while P. dissecta and P. carbophila were grouped together in a separated lineage with no significant support (Fig. 1). In the phylogeny inferred from the combined 7-gene dataset (Fig. 2), 41 species of Postia s.lat. were divided into ten monophyletic clades, and four new genera were established for monophyletic groups here. However, phylogenetic positions of four species of Postia from Argentina are not resolved because only limited gene sequences are available for them. Morphologically, P. pelliculosa and P. punctata have thick-walled, ellipsoid basidiospores that are consistent with species of Oligoporus s.str. (Rajchenberg 1987, Rajchenberg & Buchanan 1996); P. carbophila is similar to Rhodonia placenta (Rajchenberg 1995); P. dissecta is characterized by dimidiate basidiocarps with applanate pileus and cylindrical basidiospores (Rajchenberg 1987), which are similar to species of Spongiporus s.str. But their positions in the respective genera are not supported in the phylogenetic analysis (Fig. 1). For the time being, we still retain these four species in Postia s.lat.
In the current study, about 300 specimens of 41 species of Postia s.lat. had been morphologically examined, including 57 type materials (holotypes and paratypes) and many specimens from type localities, and 469 sequences had been newly obtained in this work. However, some species of Postia s.lat. were still not included in our phylogenetic analyses due to the lack of DNA sequences. For example, P. calcarea and Spongiporus cerifluus should be placed in Spongiporus s.str. because they have thin, fibrous pileus and cylindrical basidiospores; chalky basidiocarps when dry, and have hyphal pegs (Wei & Dai 2006), which are similar to species of Spongiporus s.str.; P. simanii has resupinate basidiocarps and apically encrusted cystidia, which are in line with Cystidiopostia; P. subundosa described from China resembles Osteina undosa by having hard basidiocarps with undulate pileal margin and similar sized pores (Wei & Dai 2006); P. cana, P. gloeocystidiata and P. qinensis have soft corky basidiocarps with white pore surface and allantoid basidiospores (Wei & Dai 2006, Dai et al. 2009, Yuan et al. 2010), their morphological characters are consistent with species of Postia s.str. Since no molecular data is available to support their phylogenetic positions, these species are remained in Postia s.lat.
KEY TO GENERA OF POSTIA S.LAT. IN THE ANTRODIA CLADE
1. Cystidia encrusted . . . . . . . . . . . . . . . . . . . . Cystidiopostia
1. Cystidia absent, or not encrusted if present . . . . . . . . . . . 2
2. Basidiocarps bond hard when dry, margin distinctly undulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Osteina
2. Basidiocarps corky to hard corky when dry, margin not undulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Basidiocarps resupinate . . . . . . . . . . . . . . . . . . . . . . . . . . 4
3. Basidiocarps mostly pileate, effused-reflexed . . . . . . . . . . 5
4. Basidiocarps thin and gossypine when fresh; basidiospores strongly CB+ . . . . . . . . . . . . . . . . . . . . . . . Oligoporus s.str.
4. Basidiocarps fairly thick and fleshy when fresh; basidiospores CB– . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Rhodonia
5. Basidiocarps taste bitter . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Basidiocarps taste mild . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
6. Pileal surface with circular guttulate depressions; basidiospores 2–2.5 μm wide . . . . . . . . . . . . . . . . . . . Calcipostia
6. Pileal surface glabrous; basidiospores 1.5–2 μm wide . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Amaropostia
7. Pileal surface and pore surface turning brownish when bruised . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Fuscopostia
7. Pileal surface and pore surface non-discolouring when bruised . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Basidiocarps usually imbricate; basidiospores mostly 2–3 μm wide . . . . . . . . . . . . . . . . . . . . . . . . . Spongiporus s.str.
8. Basidiocarps usually solitary; basidiospores mostly < 2 μm wide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
9. Basidiocarps more or less bluish; basidiospores weakly CB+ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Cyanosporus
9. Basidiocarps without blue tint; basidiospores CB– . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Postia s.str.
KEY TO SPECIES OF AMAROPOSTIA
1. Pores 5–6 per mm; cystidioles present . . . . . . . A. stiptica
1. Pores 7–9 per mm; cystidioles absent . . . . A. hainanensis
KEY TO SPECIES OF CYANOSPORUS
1. Basidiospores 1.4–2 μm wide . . . . . . . . . . . . . . . . . . . . 2
1. Basidiospores 0.8–1.3 μm wide . . . . . . . . . . . . . . . . . . 5
2. Cystidia present . . . . . . . . . . . . . . . . . . . . C. mongolicus
2. Cystidia absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Basidiocarps white with bright yellow margin . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. luteocaesius
3. Basidiocarps white or greyish without yellow margin . . 4
4. Pore surface becoming bluish when bruised . C. caesius
4. Pore surface unchanged when bruised . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . C. mediterraneocaesius
5. Basidiospores thin-walled, CB– 6
5. Basidiospores slightly thick-walled, CB+ . . . . . . . . . . . .7
6. Basidiocarps semicircular with azonate pileal surface; cystidioles present . . . . . . . . . . . . . . . . . . . . C. fusiformis
6. Basidiocarps ungulate with zonated pileal surface; cystidioles absent . . . . . . . . . . . . . . . . . . . . . . . . . . C. ungulatus
7. Pores 6–8 per mm . . . . . . . . . . . . . . . . . . C. microporus
7. Pores < 6 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Pileal surface glabrous . . . . . . . . . . . . . . C. subcaesius
8. Pileal surface velutinate to hirsute . . . . . . . . . . . . . . . . . 9
9. Pores 2–3 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. subhirsutus
9. Pores > 3 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
10. Pileal surface concentrically zoned by white, bluish grey and greyish brown . . . . . . . . . . . . . . . . . . . . . . C. tricolor
10. Pileal surface white to cream with blue tint . . . . . . . . . . 11
11. Growing on angiosperm wood; tramal hyphae interwoven . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. alni
11. Growing on Picea exclusively; tramal hyphae parallel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. piceicola
KEY TO SPECIES OF CYSTIDIOPOSTIA
1. Basidiospores 1.5–1.7 μm wide . . . . . . . . . . C. inocybe
1. Basidiospores < 1.5 μm wide . . . . . . . . . . . . . . . . . . . . . 2
2. Basidiocarps usually pileate; cystidia thick-walled, apically encrusted . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. pileata
2. Basidiocarps resupinate; cystidia thin-walled, smooth or apically encrusted . . . . . . . . . . . . . . . . . . . . . C. hibernica
KEY TO SPECIES OF FUSCOPOSTIA
1. Gloeocystidia present . . . . . . . . . . . . . . . . . . . . . . . . . . .2
1. Gloeocystidia absent . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2. Context duplex; basidiospores 1.8–2.5 μm wide . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F. duplicata
2. Context homogeneous; basidiospores 1–1.7 μm wide . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F. leucomallella
3. Pores 3–4 per mm; basidiospores 1.2–1.6 um wide . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F. lateritia
3. Pores 4–6 per mm; basidiospores 1.7–2.1 um wide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F. fragilis
KEY TO SPECIES OF OLIGOPORUS S.STR.
1. Pores 2–4 per mm; cystidia absent, chlamydospores present . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . O. rennyi
1. Pores 4–6 per mm; cystidia present, chlamydospores absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . O. sericeomollis
KEY TO SPECIES OF OSTEINA
1. Pores 3–5 per mm; basidiospores 2–2.5 μm wide . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . O. obducta
1. Pores 2–3 per mm; basidiospores 1–1.5 μm wide . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . O. undosa
KEY TO SPECIES OF POSTIA S.STR.
1. Pore surface grey, cream or reddish brown when dry . . . 2
1. Pore surface yellowish when dry . . . . . . . . . . . . . . . . . . . 4
2. Basidiospores broadly allantoid, 1.8–2.2 μm wide . . . . . P. lowei
2. Basidiospores narrowly allantoid, 0.8–1.5 μm wide . . . . 3
3. Basidiocarps zonate, pores 6–7 per mm; gloeoplerous hyphae absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . P. ochraceoalba
3. Basidiocarps azonate, pores 4–5 per mm; gloeoplerous hyphae present . . . . . . . . . . . . . . . . . . . . . . . . . . . P. lactea
4. Cystidia or cystidioles present . . . . . . . . . . . . . . . . . . . . . 5
4. Cystidia and cystidioles absent . . . . . . . . . . . . . . . . . . . . . 6
5. Basidiocarps small (1 × 2 × 0.5 cm), pileal surface with orange tint; grows on angiosperm wood . . . . . . P. sublowei
5. Basidiocarps big (3 × 5.5 × 1 cm), pileal surface without orange tint; grows on gymnosperm wood . . . P. amurensis
6. Pileal surface mouse-grey and hirsute; tramal hyphae thick-walled . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . P. hirsuta
6. Pileal surface cream with brown tint and tomentose; tramal hyphae thin-walled . . . . . . . . . . . . . . . . . . . P. tephroleuca
KEY TO SPECIES OF RHODONIA
1. Gloeoplerous hyphae present . . . . . . . . . . . . . . . . . . . . . . 2
1. Gloeoplerous hyphae absent . . . . . . . . . . . . . . . . . . . . . . 3
2. Basidiocarps with oblique tubes and brownish pore surface when dry; basidiospores 2–2.5 μm wide . . . . . . R. obliqua
2. Basidiocarps with straight tubes and buff pore surface when dry; basidiospores 2.5–3 μm wide . . . . . . . . . . R. placenta
3. Basidiocarps taste rancid; basidiospores larger (6–8 × 2–3 μm) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . R. rancida
3. Basidiocarps taste mild; basidiospores smaller (4.2–6 × 1.9–2.4 μm) . . . . . . . . . . . . . . . . . . . . . . . . R. subplacenta
KEY TO SPECIES OF SPONGIPORUS S.STR.
1. Pores 5–8 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Pores 2–4 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2. Cystidia present, basidiospores 2.5–3 μm wide . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. balsameus
2. Cystidia absent, basidiospores 2–2.5 μm wide . . . . . . . . 3
3. Pileal surface with grey-brown zonations; hyphal pegs absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. zebra
3. Pileal surface azonate; hyphal pegs present . S. florifomis
4. Hyphal pegs present, basidiospores allantoid (1–1.5 μm wide) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. leucospongia
4. Hyphal pegs absent, basidiospores ellipsoid (2–2.5 μm wide) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. gloeoporus
Acknowledgments
We are grateful to Dr. Zheng Wang (Yale University, USA) for improving the manuscript. We express our gratitude to the curators of H, IFP, JV, K, LY, O, PAC and TAA herbaria for the loan of specimens, to Drs. Mario Rajchenberg (CIEFAP) and Pertii Renvall (KUO) for providing specimen data. Special thanks are due to Drs. Shuang-Hui He (Beijing Forestry University, China), Hai-Sheng Yuan, Yu-Lian Wei and Li-Wei Zhou (Institute of Applied Ecology, Chinese Academy of Sciences, China) for assistance during field collections. The research was financed by the National Natural Science Foundation of China (Project Nos. 31750001 and 31670016), the Fundamental Research Funds for the Central Universities (No. 2016ZCQ04) and the National Science and Technology Foundation Project of China (No. 2014FY210400).
REFERENCES
- Bernicchia A. 2005. Polyporaceae s.l., Fungi Europaei. Candusso Alassio; Italy. [Google Scholar]
- Binder M, Hibbett DS, Larsson K-H, et al. 2005. The phylogenetic distribution of resupinate forms across the major clades of mushroom forming fungi (Homobasidiomycetes). System and Biodiversity 3: 113–157. [Google Scholar]
- Binder M, Justo A, Riley R, et al. 2013. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105: 1350–1373. [DOI] [PubMed] [Google Scholar]
- Boidin J, Mugnier J, Canales R. 1998. Taxonomie moleculaire des Aphyllophorales. Mycotaxon 66: 445–491. [Google Scholar]
- Bondartsev A, Singer R. 1941. Zur systematik der Polyporaceae. Annales Mycologici 39: 43–65. [Google Scholar]
- Brefeld O. 1888. Basidiomyceten III. Autobasidiomyceten. Untersuchungen aus dem Gesammtgebiete der Mykologie 8: 1–184. [Google Scholar]
- Buchanan PK, Ryvarden L. 2000. An annotated checklist of polypore and polypore-like fungi recorded from New Zealand. New Zealand Journal of Botany 38: 265–323. [Google Scholar]
- Chen JJ, Cui BK, Dai YC. 2016. Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 37: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Cui BK. 2016. Phylogenetic analysis and taxonomy of the Antrodia heteromorpha complex in China. Mycoscience 57: 1–10. [Google Scholar]
- Chen YY, Wu F, Wang M, et al. 2017. Species diversity and molecular systematics of Fibroporia (Polyporales, Basidiomycota) and its related genera. Mycological Progress 16: 521–533. [Google Scholar]
- Cui BK, Dai YC. 2013. Molecular phylogeny and morphology reveal a new species of Amyloporia (Basidiomycota) from China. Antonie van Leeuwenhoek 104: 817–827. [DOI] [PubMed] [Google Scholar]
- Cui BK, Li HJ. 2012. A new species of Postia (Basidiomycota) from Northeast China. Mycotaxon 120: 231–237. [Google Scholar]
- Cui BK, Vlasák J, Dai YC. 2014. The phylogenetic position of Osteina obducta (Polyporales, Basidiomycota) based on samples from northern hemisphere. Chiang Mai Journal of Science 41: 838–845. [Google Scholar]
- Dai YC. 2012. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53: 49–80. [Google Scholar]
- Dai YC, Penttilä R. 2006. Polypore diversity of Fenglin Nature Reserve, northeastern China. Annales Botanici Fennici 43: 81–96. [Google Scholar]
- Dai YC, Renvall P. 1994. Changbai wood-rotting fungi 2. Postia amylocystis (Basidiomycetes), a new polypore species. Annales Botanici Fennici 31: 71–76. [Google Scholar]
- Dai YC, Yuan HS, Wang HC, et al. 2009. Polypores (Basidiomycota) from Qin Mts. in Shaanxi Province, central China. Annales Botanici Fennici 46: 54–61. [Google Scholar]
- David A. 1980. Étude du genre Tyromyces sensu lato: répartition dans les genres Leptoporus, Spongiporus et Tyromyces sensu stricto. Bulletin Mensuel de la Société Linnéenne de Lyon 49: 6–56. [Google Scholar]
- Donk MA. 1960. The generic names proposed for Polyporaceae. Persoonia 1: 173–302. [Google Scholar]
- Donk MA. 1966. Osteina, a new genus of Polyporaceae. Schweizerische Zeit-schrift für Pilzkunde 44: 83–87. [Google Scholar]
- Donk MA. 1971. Notes on European polypores 8. Persoonia 6: 201–218. [Google Scholar]
- Erkkilä R, Niemelä T. 1986. Polypores in the parks and forests of the city of Helsinki. Karstenia 26: 1–40. [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, et al. 1994. Testing significance of incongruence. Cladistics 10: 315–319. [Google Scholar]
- Felsenstein J. 1985. Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Fries EM. 1874. Hymenomycetes Europaci. Berlingius, Lundae. [Google Scholar]
- Gilbertson RL, Ryvarden L. 1985. Some new combinations in the Polyporaceae. Mycotaxon 22: 363–365. [Google Scholar]
- Gilbertson RL, Ryvarden L. 1987. North American Polypores. 2. Megasporoporia-Wrightoporia. Fungiflora, Oslo, Norway. [Google Scholar]
- Hall TA. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Han ML, Chen YY, Shen LL, et al. 2016. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Diversity 80: 343–373. [Google Scholar]
- Han ML, Cui BK. 2015. Morphological characters and molecular data reveal a new species of Fomitopsis (Polyporales) from southern China. Mycoscience 56: 168–176. [Google Scholar]
- Hattori T, Sotome K, Ota Y, et al. 2011. Postia stellifera sp. nov., a stipitate and terrestrial polypore from Malaysia. Mycotaxon 114: 151–161. [Google Scholar]
- Hibbett DS, Donoghue MJ. 2001. Analysis of character correlations among wood decay mechanisms, mating systems, and substrate ranges in homobasidiomycetes. Systematic Biology 50: 215–242. [PubMed] [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematics and Biodiversity 42: 182–192. [Google Scholar]
- Jahn H. 1963. Mitteleuropäische Porlinge (Polyporaceae s. lato) und ihr Vorkommen in Westfalen. Westfälische Pilzbriefe 4: 1–143. [Google Scholar]
- Justo A, Hibbett DS. 2011. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 60: 1567–1583. [Google Scholar]
- Justo A, Miettinen O, Floudas D, et al. 2017. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. [DOI] [PubMed] [Google Scholar]
- Jülich W. 1982. Notes on some Basidiomycetes (Aphyllophorales and Heterobasidiomycetes). Persoonia 11: 421–428. [Google Scholar]
- Karsten PA. 1881. Enumeratio boletinearum et polyporearum fennicarum, systemate novo dispositarum. Revue Mycologique Toulouse 3: 16–19. [Google Scholar]
- Kim CS, Jo JW, Kwag YN, et al. 2015. Mushroom flora of Ulleung-gun and a newly recorded Bovista species in the Republic of Korea. Mycobiology 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Lee JS, Jung HS. 2007. Fomitopsis incarnatus sp. nov. based on generic evaluation of Fomitopsis and Rhodofomes. Mycologia 99: 833–841. [DOI] [PubMed] [Google Scholar]
- Kim KM, Park SY, Jung HS. 2001. Phylogenetic classification of Antrodia and related genera based on ribosomal RNA internal transcribed space sequences. Journal of Microbiology and Biotechnology 11: 475–481. [Google Scholar]
- Larsen MJ, Lombard FF. 1986. New combinations in the genus Postia Fr. (Polyporaceae). Mycotaxon 26: 271–273. [Google Scholar]
- Li HJ, Cui BK, Dai YC. 2014. Taxonomy and multi-gene phylogeny of Datronia (Polyporales, Basidiomycota). Persoonia 32: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner DL, Banik MT. 2008. Molecular phylogeny of Laetiporus and other brown rot polypore genera in North America. Mycologia 100: 417–430. [DOI] [PubMed] [Google Scholar]
- Lowe JL. 1975. Polyporaceae of North America. The genus Tyromyces. Mycotaxon 2: 1–82. [Google Scholar]
- Matheny PB. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, et al. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Wang Z, Binder M, et al. 2007. Contributions of RPB2 and TEF1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–451. [DOI] [PubMed] [Google Scholar]
- McGinty NJ. 1909. A new genus, Cyanosporus. Mycological Notes 33: 436. [Google Scholar]
- Murrill WA. 1905. The Polyporaceae of North America 12. A synopsis of the white and bright-colored pileate species. Bulletin of the Torrey Botanical Club 32: 469–493. [Google Scholar]
- Murrill WA. 1907. Polyporaceae 1. North American Flora 9: 1–72. [Google Scholar]
- Murrill WA. 1912. Polyporaceae and Boletaceae of the Pacific Coast. Mycologia 4: 91–100. [Google Scholar]
- Niemelä T. 2005. Polypores, lignicolous fungi. Norrlinia 13: 1–320. [Google Scholar]
- Niemelä T, Dai YC, Kinnunen J, et al. 2004. New and in North Europe rare polypore species (Basidiomycota) with annual, monomitic basidiocarps. Karstenia 44: 67–77. [Google Scholar]
- Niemelä T, Kinnunen J, Larsson KH, et al. 2005. Genus revision and new combinations of some north European polypores. Karstenia 45: 75–80. [Google Scholar]
- Niemelä T, Penttilä R, Kinnunen J, et al. 2001. Novelties and records of poroid Basidiomycetes in Finland and adjacent Russia. Karstenia 41: 1–21. [Google Scholar]
- Núñez M, Ryvarden L. 2001. East Asian polypores 2. Synopsis Fungorum 14: 165–522. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Ortiz-Santana B, Lindner DL, Miettinen O, et al. 2013. A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales). Mycologia 105: 1391–1411. [DOI] [PubMed] [Google Scholar]
- Page RMD. 1996. Treeview: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Papp V. 2014. Nomenclatural novelties in the Postia caesia complex. Mycotaxon 129: 407–413. [Google Scholar]
- Petersen JH. 1996. Farvekort. The Danish Mycological Society’s color-chart. Foreningen til Svampekundskabens Fremme, Greve. [Google Scholar]
- Pieri M, Rivoire B. 2005. Postia mediterraneocaesia, une nouvelle espèce de polypore découverte dans le sud de l’Europe. Bulletin Semestriel de la Fédération des Associations Mycologiques Méditerranéennes 28: 33–38. [Google Scholar]
- Pildain MB, Rajchenberg M. 2013. The phylogenetic position of Postia s.l. (Polyporales, Basidiomycota) from Patagonia, Argentina. Mycologia 105: 357–367. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rajchenberg M. 1987. Xylophilous (Aphyllophorales, Basidiomycetes) from the southern Andean forests. Sydowia 40: 235–249. [Google Scholar]
- Rajchenberg M. 1995. New polypores from the Nothofagus forests of Argentina. Mycotaxon 54: 427–453. [Google Scholar]
- Rajchenberg M, Buchanan PK. 1996. Two newly described polypores from Australasia and southern South America. Australian Systematic Botany 9: 877–885. [Google Scholar]
- Rajchenberg M, Gorjón SP, Pildain MB. 2011. The phylogenetic disposition of Antrodia s.l. (Polyporales, Basidiomycota) from Patagonia Argentina. Australian Systematic Botany 24: 111–120. [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Renvall P. 1992. Basidiomycetes at the timberline in Lapland 4. Postia lateritia n. sp. and its rust-coloured relatives. Karsternia 32: 43–60. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ryvarden L. 1981. Type studies in the Polyporaceae13. Species described by J.H. Léveillé. Mycotaxon 13: 175–186. [Google Scholar]
- Ryvarden L. 1991. Genera of polypores, nomenclature and taxonomy. Synopsis Fungorum 5: 1–363. [Google Scholar]
- Ryvarden L, Gilbertson RL. 1994. European polypores 2. Synopsis Fungorum 7: 394–743. [Google Scholar]
- Ryvarden L, Melo I. 2014. Poroid fungi of Europe. Synopsis Fungorum 31: 1–455. [Google Scholar]
- Shen LL, Cui BK. 2014. Morphological and molecular evidence for a new species of Postia (Basidiomycota) from China. Cryptogamie Mycologie 35: 199–207. [Google Scholar]
- Shen LL, Cui BK, Dai YC. 2014. A new species of Postia (Polyporales, Basidiomycota) from China based on morphological and molecular evidence. Phytotaxa 162: 147–156. [Google Scholar]
- Shen LL, Liu HX, Cui BK. 2015. Morphological characters and molecular data reveal two new species of Postia (Basidiomycota) from China. Mycological Progress 14: 7. [Google Scholar]
- Song J, Chen JJ, Wang M, et al. 2016. Phylogeny and biogeography of the remarkable genus Bondarzewia (Basidiomycota, Russulales). Scientific Reports 6: 34568. doi: https:/doi.org/10.1038/srep34568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Chen YY, Cui BK, et al. 2014. Morphological and molecular evidence for two new species of Laetiporus (Basidiomycota, Polyporales) from southwestern China. Mycologia 106: 1039–1050. [DOI] [PubMed] [Google Scholar]
- Song J, Cui BK. 2017. Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evolutionary Biology 17: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin V. 2016. Taxonomy and phylogeny of brown-rot fungi in the Antrodia complex (Polyporales, Basidiomycota). PhD thesis, University of Helsinki, Finland, Helsinki. [Google Scholar]
- Spirin V, Miettinen O, Pennanen J, et al. 2013a. Antrodia hyalina, a new polypore from Russia, and A. leucaena, new to Europe. Mycological Progress 12: 53–61. [Google Scholar]
- Spirin V, Runnel K, Vlasák J, et al. 2015. Species diversity in the Antrodia crassa group (Polyporales, Basidiomycota). Fungal Biology 119: 1291–1310. [DOI] [PubMed] [Google Scholar]
- Spirin V, Vlasák J, Miettinen O. 2016a. Studies in the Antrodia serialis group (Polyporales, Basidiomycota). Mycologia 109: 217–230. [DOI] [PubMed] [Google Scholar]
- Spirin V, Vlasák J, Niemelä T, et al. 2013b. What is Antrodia sensu stricto? Mycologia 105: 1555–1576. [DOI] [PubMed] [Google Scholar]
- Spirin V, Vlasák J, Rivoire B, et al. 2016b. Hidden diversity in the Antrodia malicola group (Polyporales, Basidiomycota). Mycological Progress 15: 1–12. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analysis with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, et al. 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Symposium Series 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ţura D, Spirin WA, Zmitrovich IV, et al. 2008. Polypores new to Israel 1: genera Ceriporiopsis, Postia and Skeletocutis. Mycotaxon 103: 217–227. [Google Scholar]
- Vampola P, Ordynets A, Vlasak J. 2014. The identity of Postia lowei (Basidiomycota, Polyporales) and notes on related or similar species. Czech Mycology 66: 39–52. [Google Scholar]
- Walker J. 1996. An opinion on the validity of the generic name Postia Fries 1874 (Eumycota: Aphyllophorales). Australasian Mycological Society Newsletter 15: 23–26. [Google Scholar]
- Wei YL, Dai YC. 2006. Three new species of Postia (Aphyllophorales, Basidiomycota) from China. Fungal Diversity 23: 391–402. [Google Scholar]
- Wei YL, Qin WM. 2010. Two new species of Postia from China. Sydowia 62: 165–170. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego. [Google Scholar]
- Yao YJ, Pegler DN, Chase MW. 2005. Molecular variation in the Postia caesia complex. FEMS Microbiology Letters 242: 109–116. [DOI] [PubMed] [Google Scholar]
- Yuan HS, Dai YC, Wei YL. 2010. Postia cana sp. nov. (Basidiomycota, Polyporales) from Shanxi Province, northern China. Nordic Journal of Botany 28: 629–631. [Google Scholar]
- Zhao CL, Cui BK, Song J, et al. 2015. Fragiliporiaceae, a new family of Polyporales (Basidiomycota). Fungal Diversity 70: 115–126. [Google Scholar]
- Zhou JL, Zhu L, Chen H, et al. 2016. Taxonomy and phylogeny of Polyporus group Melanopus (Polyporales, Basidiomycota) from China. Plos One 11 (8): e0159495. doi: 10.1371/journal.pone.0159495. [DOI] [PMC free article] [PubMed] [Google Scholar]


