Abstract
A section-based taxonomy of Cortinarius, covering large parts of the temperate North and South Hemispheres, is presented. Thirty-seven previously described sections are reviewed, while another forty-two sections are proposed as new or as new combinations. Twenty additional clades are recovered but not formally described. Furthermore, six new or combined species names are introduced, and one species is neotypified. The structure is supported by morphological characters and molecular evidence, based on two (nrITS and nrLSU) and four (nrITS, nrLSU, rpb1 and rpb2) loci datasets and analysed by Maximum Likelihood methods (PhyML, RAxML). Altogether 789 Cortinarius samples were included in the study.
Keywords: Basidiomycota, Maximum Likelihood, phylogeny, ribosomal and protein-coding genes, section rank, Southern Hemisphere
INTRODUCTION
It is self-evident that large fungal genera are in a special need for structuring into lower-rank taxa in order to assist the mycologist in navigating the genus and to provide an overview of its taxonomy. Cortinarius is the most diverse and species-rich genus of macrofungi (cf. Niskanen et al. 2016). Historically, several systems of subgenera, sections, and other infrageneric taxa were erected in Cortinarius, based on the macromorphology of geographically limited samplings. These taxa were in many cases emended, combined, or divided as micromorphological, chemical, and later molecular data became available from a widening geographical span. In his ground-breaking work, Moser in Singer (1986) listed all then known sections and other supraspecific taxa of the genus, a base that was later used to expand the taxonomy in different directions (e.g., Bidaud et al. 1994, Brandrud et al. 1994: 31). We expect this process to continue, especially when considering that many geographical areas (e.g., Africa) remain poorly sampled, and will no doubt prove to contain additional Cortinarius taxa.
Many studies during the past twenty years have explored different aspects of the phylogeny of Cortinarius. Most of the species are described from Europe (c. 1 900 out of a total 2 700 worldwide), followed by North America, which means that the Northern Hemisphere tends to dominate in extant works (cf. Peintner et al. 2004). But in their barcoding study, Garnica et al. (2016) addressed the genus on a global scale and revealed a cladal structure of c. 900 species based on the internal transcribed spacer regions (nrITS) of the nuclear ribosomal DNA. Garnica et al. (2016: Fig. S2) also produced a phylogram of a limited sampling based on five loci, annotated with support figures. This showed that the genus contains two major lineages that appear to be endemic to the Northern Hemisphere, namely sect. Calochroi and subg. Telamonia s.str., thus corroborating previous works on these particular groups (Høiland & Holst-Jensen 2000, Frøslev et al. 2006b, Niskanen 2008, Ortega et al. 2008, Garnica et al. 2009, 2011, Niskanen et al. 2012, cf. Soop & Gasparini 2011). In addition, several other works provide the outline of a phylogeny-based infrageneric taxonomy for selected groups within the genus, based on northern taxa (Brandrud et al. 2013, 2014, Liimatainen et al. 2014, Saar et al. 2014).
Many studies during the past twenty years have explored different aspects of the phylogeny of Cortinarius. Most of the species are described from Europe (c. 1 900 out of a total 2 700 worldwide), followed by North America, which means that the Northern Hemisphere tends to dominate in extant works (cf. Peintner et al. 2004). But in their barcoding study, Garnica et al. (2016) addressed the genus on a global scale and revealed a cladal structure of c. 900 species based on the internal transcribed spacer regions (nrITS) of the nuclear ribosomal DNA. Garnica et al. (2016: Fig. S2) also produced a phylogram of a limited sampling based on five loci, annotated with support figures. This showed that the genus contains two major lineages that appear to be endemic to the Northern Hemisphere, namely sect. Calochroi and subg. Telamonia s.str., thus corroborating previous works on these particular groups (Høiland & Holst-Jensen 2000, Frøslev et al. 2006b, Niskanen 2008, Ortega et al. 2008, Garnica et al. 2009, 2011, Niskanen et al. 2012, cf. Soop & Gasparini 2011). In addition, several other works provide the outline of a phylogeny-based infrageneric taxonomy for selected groups within the genus, based on northern taxa (Brandrud et al. 2013, 2014, Liimatainen et al. 2014, Saar et al. 2014).
On the other hand, it is evident from the cited works, as well as from other studies (Peintner et al. 2004, Garnica et al. 2005, Stensrud et al. 2014, Soop 2016, Soop et al. 2018), that the genus contains many lineages that are shared between the Northern and Southern Hemispheres, as well as others that appear to be endemic to either. They are often also widely distributed within their hemispheres; for example, a substantial number of clades are shared between North America and Europe (Garnica et al. 2011, Harrower et al. 2011, Niskanen et al. 2012, Ammirati et al. 2013, Liimatainen et al. 2015). One notes, however, that so far little has been done on the Cortinarius taxonomy in north-eastern Asia and in Africa, leaving an important gap in our knowledge of the genus (cf. Horak 1983).
A common result from many of the cited phylogenetic studies, is that most traditional subgenera (such as Phlegmacium and Telamonia) turn out to be polyphyletic, while many smaller, lower-rank taxa look promising for structuring the genus from well-supported monophyletic clades. The phylogenetic delineation of well-supported subgenera within Cortinarius remains to be achieved through the sequencing of additional genes, or more promisingly, using a phylogenomics approach. In the interim, sections suggest themselves as suitable, monophyletic building-blocks, that may be used later to construct higher taxa. Consequently, in this study we aimed at the following:
– Combine morphological markers with suitable genetic markers to map the sections of the genus, based on as large a sample set as possible.
– Use existing sections or other suitable taxa as far as possible, sometimes in the form of new combinations. When not possible, describe new sections.
– Provide, for each proposed section, a list of species, either species sampled in the study, or putative species that we assume to be members.
– Map out the geographical distribution of Cortinarius sections, with particular attention to the Southern Hemisphere, an area that has so far been sparingly studied (Horak 1983).
With this approach to a supraspecific taxonomy, based on a large number of globally sampled species, we hope to provide a useful framework for expanding the taxonomy of the genus, into higher ranks (e.g., subgenera) or lower ranks (e.g., subsections), as further supported clades become apparent. Thus, in a future effort the sections may be combined or divided, or they may form the basis for new combinations.
MATERIALS AND METHODS
Geographical scope
As mentioned in the Introduction, due to scarcity of material, African and Northeast-Asian species are grossly underrepresented. The following principal areas have been sampled: Europe, North/Central/South America, Australia, and New Zealand.
Taxonomic scope
All the samples are specimens of Cortinarius s.lat., including the genera Cuphocybe, Protoglossum, Quadrispora, Rapacea, Rozites, Thaxterogaster, and Hymenogaster p.p., these being synonyms of Cortinarius (Peintner et al. 2001, 2002, Gasparini 2013, 2016). We also include the genus Gigasperma. Two important boreal groups, Calochroi and Telamonia s.str., are represented only by a few token species, due to several recent and ongoing studies cited in the Introduction, which explore the infrageneric ranks involved.
Molecular sampling
Sequences from 634 collections were chosen from GenBank (http://www.ncbi.nlm.nih.gov/) or UNITE (http://unite.ut.ee/), and another 346 sequences were newly generated in this study (Table 1). For a detailed description of the methods used for DNA extraction and PCRs see Soop et al. (2016) and Papp & Dima (2018). Where possible, type collections were included in the dataset; 140 samples represent holo-, neo-, epi- or paratypes. All samples were sequenced in the nrITS (ITS1+5.8S+ITS2) region, and in addition most were sequenced in one or more of the nrLSU, rpb1, and rpb2 regions. Seventy collections are represented only by ITS; in these cases the taxon was considered important to confirm a position in the phylogeny. When many sequences of a species were available and their similarity in a separate alignment (not shown) was > 99 %, only one or two samples were chosen. Three species in genera Conocybe, Descolea, and Flammula were chosen as outgroup. See Table 1 for GenBank and fungarium voucher numbers, sections, and provenance.
Table 1.
Sequences newly generated and first published in this study.
| Species | Herbarium ID | GenBank accession no. |
Section/Clade | Country | |||
|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb1 | rpb2 | ||||
| C. achrous cf. | PDD107722/CO2192 | KT875175 | in ITS | /Achroi | New Zealand | ||
| C. aegrotus (type) | PDD27270 | GU233389 | New Zealand | ||||
| C. aerugineoconicus (type) | PDD27258 | GU233408 | /Entheosi | New Zealand | |||
| C. alboaggregatus | JAC12509/PDD96523 | MH101554 | MH108393 | MH141038 | Alboaggregati | New Zealand | |
| C. alboamarescens | TEB334-14/DB5405 | MK358079 | Vibratiles | Norway | |||
| C. alboroseus | PDD105432/JAC13150 | MH108404 | New Zealand | ||||
| C. alienatus | PDD27180 | GU233384 | New Zealand | ||||
| PDD96972/JAC12868 | MH101562 | MH108401 | New Zealand | ||||
| C. amblyonis cf. | PDD94049/CO1801 | MH101544 | MH108383 | Obtusi | New Zealand | ||
| C. anisodorus | PDD88506 | KT334133 | KT334145 | New Zealand | |||
| C. ardesiacus cf. | PDD72855 | MH101533 | MH108374 | /Ardesiaci | New Zealand | ||
| C. areni-silvae | MIKH-T508 | MK358080 | MK358059 | MK340950 | Phlegmacioides | Russia | |
| C. areolatoimbricatus | PSC1552 | MK358081 | MK340951 | MK340969 | Australia | ||
| C. armiae | PDD105600/JAC13347 | MH101568 | MH108406 | Limonii | New Zealand | ||
| C. atrolazulinus | PDD97542/CO1917 | KJ635241 | in ITS | New Zealand | |||
| C. australiensis | PERTH 6434991 | MG553064 | MK340952 | MK340970 | /Australienses | Australia | |
| C. australiensis cf. | JAC12796 | MK358082 | MK358060 | MK340953 | MK340971 | /Australienses | New Zealand |
| C. australis | PDD107712/CO2182 | KT875192 | in ITS | Purpurascentes | New Zealand | ||
| PDD80010/JAC8617 | MH101535 | MH108375 | Purpurascentes | New Zealand | |||
| C. austrovaginatus | PDD80251/JAC8985 | MH101537 | MH108377 | Austrovaginati | New Zealand | ||
| PDD94052/CO1808 | MK358083 | Austrovaginati | New Zealand | ||||
| C. badiohepaticus ined. | PDD72785 | MH101530 | MH108364 | Lustrabiles | New Zealand | ||
| C. balteatibulbosus | SSt16-073 | MK358084 | MK358061 | MK340954 | Phlegmacioides | Germany | |
| C. barbatus | TEB582b-15 | MK358085 | Vibratiles | Norway | |||
| C. basifibrillosus ined. | PDD72794 | MH101531 | MH108368 | Obtusi | New Zealand | ||
| C. bellus | PDD103880/CO1238 | KF727319 | New Zealand | ||||
| C. brunneotinctus | DB6257 | MK358086 | MK358062 | MK340955 | Hungary | ||
| C. brunneus | DB2548 | MK358063 | Telamonia | Hungary | |||
| C. caesiostramineus | DB6237 | MK340956 | Caerulescentes | Norway | |||
| C. calaisopus | PDD103678/CO2106 | KF727338 | Delibuti | New Zealand | |||
| C. calaisopus II | PDD80264/JAC8990 | MH101538 | Delibuti | New Zealand | |||
| C. camptoros | GS16-5 | MK358087 | MK340957 | /Camptori | Germany | ||
| C. carbonellus (type) | PDD70502/CO1045 | GU233391 | MH141041 | MH141021 | Carbonelli | New Zealand | |
| C. cardinalis (type) | PDD27174 | GU233415 | New Zealand | ||||
| C. carneipallidus | PDD103682/CO2110 | KF727337 | Cortinarius | New Zealand | |||
| C. cartilagineus | PDD105768/JAC13517 | MH108409 | /Cartilaginei | New Zealand | |||
| C. caryotis (type) | PDD71004/CO1043 | GU233407 | MH141039 | Limonii | New Zealand | ||
| C. caryotoides | PDD105781/JAC13530 | MH101572 | MH108410 | Limonii | New Zealand | ||
| C. castaneiceps (type) | PDD27269 | GU233332 | New Zealand | ||||
| C. castaneiceps cf. | PDD106108/JAC13905 | MH101580 | MH108418 | Malvacaei | New Zealand | ||
| C. castaneodiscus | PDD72712 | MH101525 | MH108347 | Ignelli | New Zealand | ||
| C. castaneodiscus II | PDD107509/CO1236 | MG019348 | MG019374 | Ignelli | New Zealand | ||
| C. castoreus | JAC12825/PDD96929 | MH101557 | MH108396 | MH141045 | Rapacea | New Zealand | |
| C. chlorophyllus (type) | PDD103681/CO2109 | KF727327 | Scauri | New Zealand | |||
| C. chrysma (type) | PDD68469/CO788 | GU233393 | Chrysmata | New Zealand | |||
| F44428/CO1234 | MK358088 | MK358064 | MK340958 | Chrysmata | New Zealand | ||
| C. chrysoconius cf. | PDD105532/JAC13280 | MH101567 | MH108405 | /Chrysoconii | New Zealand | ||
| C. collybianus (type) | PDD70509/CO1074 | GU233417 | MH141024 | Callistei | New Zealand | ||
| C. collybianus cf. | PDD72676 | MH101523 | Callistei | New Zealand | |||
| C. conei | PDD83709/JAC9578 | MH101539 | Austrovaginati | New Zealand | |||
| C. cramesinus (type) | PDD27173 | GU233420 | New Zealand | ||||
| C. cremeolina (type) | PDD70506/CO1058 | JX000380 | Cremeolinae | New Zealand | |||
| C. cremeolina cf. | PDD105601/JAC13348 | MH101569 | MH108407 | Cremeolinae | New Zealand | ||
| C. cremeolina var. subpicoides | PDD105782/JAC13531 | MH108411 | Cremeolinae | New Zealand | |||
| PDD107719/CO2189 | KT875196 | in ITS | Cremeolinae | New Zealand | |||
| C. cruentoides (type) | PDD101864/CO2038 | MH141051 | MH141014 | Cruentoides | New Zealand | ||
| C. crypticus (type) | PDD27002 | JQ063072 | Gigasperma | New Zealand | |||
| PDD100127 | JQ063070 | JQ063071 | Gigasperma | New Zealand | |||
| C. cucumeris | PDD96335/JAC12095 | MH108392 | Cycnei | New Zealand | |||
| C. cuphomorphus (type) | PDD103680/CO2108 | KF727317 | Cuphomorphi | New Zealand | |||
| C. cupreonatus (type) | PDD70503/CO1048 | JX000379 | New Zealand | ||||
| JAC13774/PDD105979 | MH101577 | MH108415 | MH141040 | MH141020 | New Zealand | ||
| C. cycneus | PDD103783 | MH101565 | MH108403 | Cycnei | New Zealand | ||
| C. cypripedi (type) | PDD107723/CO2193 | MH141050 | Illumini | New Zealand | |||
| C. daulnoyae | SSt15-097 | MK358089 | MK358065 | MK340959 | Phlegmacioides | Germany | |
| C. diaphorus ined. | PDD107503/CO1447 | MG019351 | MG019370 | New Zealand | |||
| C. dulciolens | FUNNZ2013-26 | MK358090 | MK358066 | Dulciolentes | New Zealand | ||
| C. dulciorum (type) | PDD78797/CO1460 | JX000395 | Cremeolinae | New Zealand | |||
| C. durifoliorum | PDD107700/CO2170 | MH141033 | MH141028 | Anomali | New Zealand | ||
| C. dysodes (type) | PDD70499/CO1038 | GU233394 | Camphorati | New Zealand | |||
| C. dysodes cf. | PDD96310/JAC12070 | MH101551 | MH108390 | Camphorati | New Zealand | ||
| C. elaiops (type) | PDD88271/CO1649 | JX000400 | Pauperae | New Zealand | |||
| C. emollitoides | DB1576 | MK358091 | Vibratiles | Hungary | |||
| C. eunomalus | PDD107706/CO2176 | MH141035 | MH141029 | New Zealand | |||
| C. eutactus (type) | PDD78807/CO1483 | JX000397 | Crassi | New Zealand | |||
| C. exlugubris (type) | PDD67181/CO818 | GU233409 | New Zealand | ||||
| C. fasciatus cf. | TEB517-15/DB5839 | MK358092 | MK358067 | MK340960 | Laeti | Norway | |
| C. faucium ined. | PDD94046/CO1795 | KP343698 | KP343699 | /Rufoaurantii | New Zealand | ||
| C. georgiolens | GS03-1 | MK358093 | MK358068 | MK340961 | Caerulescentes | Germany | |
| C. icterinoides | CO1690 | MK358094 | MK340962 | Chrysmata | New Zealand | ||
| C. ignellus (type) | PDD73154/CO1245 | JX000390 | Ignelli | New Zealand | |||
| PDD103698/CO2123 | KF727313 | Ignelli | New Zealand | ||||
| C. incensus (type) | PDD73147/CO1225 | MK358095 | JX000387 | Incensi | New Zealand | ||
| C. indolicus | PDD103881/CO1246 | KF727334 | New Zealand | ||||
| C. indotatus | PDD107733/CO2203 | KT875182 | in ITS | Pauperae | New Zealand | ||
| C. ionomataius (type) | PDD78765/CO1406 | JX000393 | New Zealand | ||||
| PDD80011/JAC8615 | MH101536 | MH108376 | New Zealand | ||||
| C. ixomolynus | PDD107720/CO2190 | KT875207 | in ITS | New Zealand | |||
| C. kaimanawa (type) | PDD73133/CO1259 | JX000383 | Purpurascentes | New Zealand | |||
| PDD101841/CO2014 | KJ635213 | in ITS | Purpurascentes | New Zealand | |||
| C. laetiluteinus ined. | PDD101852/CO2025 | KJ635215 | in ITS | Laeti | New Zealand | ||
| C. lamproxanthus (type) | PDD78780/CO1429 | JX000394 | New Zealand | ||||
| C. laquellus | PDD72766 | MH101527 | MH108355 | Laquelli | New Zealand | ||
| C. leptospermorum (type) | PDD27183 | GU233395 | Pauperae | New Zealand | |||
| C. lubricanescens | PDD75709 | GU233402 | Cycnei | New Zealand | |||
| PDD95404/JAC10948 | MH101546 | MH108385 | Cycnei | New Zealand | |||
| C. luteinus (type) | PDD73137/CO1257 | JX000386 | Luteini | New Zealand | |||
| C. mariae | PDD72487 | MH101518 | MH108318 | MH141044 | Rapacea | New Zealand | |
| C. marmoratus | PDD71007/CO1014 | GU233381 | GU233381 | Marmorati | New Zealand | ||
| C. medioscaurus (type) | PDD103691/CO2121 | KF727332 | Austrovaginati | New Zealand | |||
| C. meleagris | PDD72781 | HM060323 | Rozites | New Zealand | |||
| PDD96207/JAC11811 | MH101549 | MH108388 | Rozites | New Zealand | |||
| C. melimyxa | PDD94024/CO1768 | GU233405 | New Zealand | ||||
| C. melleomitis | PDD107704/CO2174 | KT875184 | in ITS | MH141043 | MH141026 | Vibratiles | New Zealand |
| C. memoria-annae | JAC8614 | MK358096 | MK358069 | New Zealand | |||
| C. minorisporus ined. | PDD95306/JAC10838 | KT334129 | KT334142 | New Zealand | |||
| C. minoscaurus (type) | PDD71005/CO1013 | GU233377 | New Zealand | ||||
| PDD87013/JAC9904 | MH101540 | MH108379 | New Zealand | ||||
| C. miwok | CO610 | MK358097 | Telamonia | USA | |||
| C. mycenarum (type) | PDD107715/CO2185 | MH141048 | MH141013 | New Zealand | |||
| C. myrticaryotis ined. | PDD103635/CO815 | KF727388 | KF727339 | Limonii | New Zealand | ||
| C. naphthalinus (type) | PDD70505/CO1054 | GU233401 | New Zealand | ||||
| C. napivelatus | PDD72728 | MH108348 | Subcastanelli | New Zealand | |||
| C. neocallisteus | CO2145 | MK358098 | Callistei | Sweden | |||
| C. olidoamarus | DB6012 | MK358099 | MK340963 | MK340972 | Glaucopodes | Hungary | |
| C. olivaceoniger | PDD96938/JAC12834 | MH101558 | MH108397 | MH141049 | MH141012 | Walkeri | New Zealand |
| C. olivaceopictus cf. | JAC12554/PDD96679 | MH101556 | MH108395 | MH141047 | MH141011 | Pauperae | New Zealand |
| C. olorinatus | PDD72753 | HM060331 | New Zealand | ||||
| C. ophryx (type) | PDD78769/CO1411 | KJ547667 | Persplendidi | New Zealand | |||
| C. ophryx cf. | PDD103688/CO2117 | MK358100 | Persplendidi | New Zealand | |||
| C. orixanthus (type) | PDD88253/CO1614 | JX000398 | /Orixanthi | New Zealand | |||
| C. papaver (type) | PDD71003/CO1066 | GU233399 | New Zealand | ||||
| C. paraonui (type) | PDD77471/CO1316 | JX000392 | New Zealand | ||||
| C. paraxanthus (type) | PDD78802/CO1472 | JX000396 | Paraxanthi | New Zealand | |||
| C. peraureus (type) | PDD67177/CO785 | JX000378 | New Zealand | ||||
| PDD103638/CO1047 | KF727391 | KF727321 | New Zealand | ||||
| C. peraurilis | PDD103660/CO2087 | MH101564 | MK340964 | New Zealand | |||
| C. perelegans (type) | PDD70500/CO1040 | GU233398 | New Zealand | ||||
| C. periclymenus (type) | PDD71008/CO1060 | GU233379 | New Zealand | ||||
| C. persplendidus (type) | PDD27168 | GU233387 | Persplendidi | New Zealand | |||
| PDD96608/JAC12491 | MH101555 | MH108394 | MH141052 | MH141017 | Persplendidi | New Zealand | |
| C. phaeomyxa | PDD107511/CO1025 | MG019367 | Cuphocybe | New Zealand | |||
| C. pholiotellus | PDD96959/JAC12855 | MH101560 | MH108399 | MH141042 | MH141016 | New Zealand | |
| PDD96960/JAC12856 | MH101561 | MH108400 | New Zealand | ||||
| C. picoides | PDD103886/CO1643 | KF727302 | Turmales | New Zealand | |||
| PDD94019 | GU233371 | GU233424 | MH141037 | MH141022 | Turmales | New Zealand | |
| C. pisciodorus | JAC13813/PDD106018 | MH108417 | MH141031 | Dulciolentes | New Zealand | ||
| C. poliotrichus ined. | PDD103684/CO2112 | KF727390 | KF727333 | Austrocyanites | New Zealand | ||
| C. porphyroideus | CO1663 | MK358102 | New Zealand | ||||
| C. porphyrophaeus (type) | PDD27263 | GU233331 | GU233416 | New Zealand | |||
| C. promethenus (type) | PDD94059/CO1815 | MK340965 | New Zealand | ||||
| C. pseliocaulis | PDD105646/JAC13394 | MH101570 | MH108408 | New Zealand | |||
| C. pseudoarcuatorum | TEB584-16 | MK358103 | MK340966 | Russia | |||
| C. pyrrhomarmarus ined. | PDD78789/CO1449 | MK358104 | Austroduracini | New Zealand | |||
| C. rattinoides (type) | PDD88283/CO1673 | JX000406 | Anomali | New Zealand | |||
| C. rattinus (type) | PDD71009/CO1061 | GU233419 | Carbonelli | New Zealand | |||
| C. reverendissimus | TEB630-16 | MK358105 | MK358071 | MK340967 | /Varii | Russia | |
| C. rhipiduranus (type) | PDD88269/CO1645 | JX000399 | Purpurascentes | New Zealand | |||
| PDD103673/CO2101 | KF727323 | Purpurascentes | New Zealand | ||||
| C. rotundisporus | PDD96298/JAC12057 | MH101550 | MH108389 | Delibuti | New Zealand | ||
| C. rotundisporus cf. | PDD72733 | MH101526 | MH108349 | Delibuti | New Zealand | ||
| C. rubripurpuratus | PDD103883/CO1453 | KF727306 | New Zealand | ||||
| C. rubrodactylus | PDD105784/JAC13533 | MH101574 | MH108412 | Callistei | New Zealand | ||
| C. saturniorum (type) | PDD67176/CO783 | GU233388 | New Zealand | ||||
| JAC13780/PDD105985 | MH101578 | MH108416 | MH141034 | MH141019 | New Zealand | ||
| C. sciurellus (type) | PDD103641/CO1679 | KF727303 | MH141015 | Pauperae | New Zealand | ||
| C. sclerophyllorum cf. | PDD72685/ZT9610 | MH101524 | MH108339 | Bolares | New Zealand | ||
| C. singularis cf. | PDD103675/CO2103 | KF727376 | KF727326 | Scauri | New Zealand | ||
| C. singularis II cf. | PDD72665 | MH101521 | MH108335 | Scauri | New Zealand | ||
| C. sp. | PDD96951/JAC12847 | MH101559 | MH108398 | Verniciori | New Zealand | ||
| C. sp. | PDD97072/JAC12973 | MH101563 | MH108402 | Pauperae | New Zealand | ||
| C. sp. | Buyck 08-153 | MK358106 | MK358072 | Cortinarius | Madagascar | ||
| C. sp. | Buyck 08-252 | MK358107 | MK358073 | Persplendidi | Madagascar | ||
| C. sp. I | PDD72770 | MH101528 | MH108357 | Laeti | New Zealand | ||
| C. sp. I | PDD107520/CO1319 | MK358108 | /Minilaci | New Zealand | |||
| C. sp. I | PDD87652/JAC10807 | MH101542 | MH108381 | Obtusi | New Zealand | ||
| C. sp. II | PDD72798/ZT9699 | MH101532 | MH108370 | /Minilaci | New Zealand | ||
| C. sp. II | PDD72773 | MH101529 | MH108359 | Obtusi | New Zealand | ||
| C. sp. III | PDD87651/JAC10806 | MH101541 | MH108380 | Laeti | New Zealand | ||
| C. sp. III | JAC12593 | MK358109 | MK358074 | Obtusi | New Zealand | ||
| C. sp. IV | PDD87682/JAC10674 | MH101543 | MH108382 | Laeti | New Zealand | ||
| C. sp. IV | JAC13734 | MK358110 | Obtusi | New Zealand | |||
| C. sp. V | PDD72670 | MH101522 | Laeti | New Zealand | |||
| C. sp. V | PDD95246/JAC10673 | MH101545 | MH108384 | MH141046 | MH141010 | Obtusi | New Zealand |
| C. squameopercomis ined. | TEB397-16 | MK358111 | MK358075 | Percomes | Norway | ||
| C. subcastanellus | PDD95557/JAC11107 | MH108386 | Subcastanelli | New Zealand | |||
| FUNNZ2013 1219 | MK358112 | MK358076 | Subcastanelli | New Zealand | |||
| C. subgemmeus | PDD72620 | MH101520 | MH108325 | Rubicunduli | New Zealand | ||
| PDD78793/CO1455 | MH101534 | Rubicunduli | New Zealand | ||||
| C. suborixanthus ined. | PDD101824/CO1994 | KJ635208 | in ITS | /Orixanthi | New Zealand | ||
| C. suecicolor (type) | PDD74698/CO1185 | JX000391 | Anomali | New Zealand | |||
| C. suecicolor cf. | PDD105967/JAC13762 | MH101576 | MH108414 | MH141032 | MH141027 | Anomali | New Zealand |
| C. taylorianus cf. | PDD107692/CO2162 | MH101581 | MH108419 | Archeriani | New Zealand | ||
| C. tessiae | PDD107517/CO1450 | MG019356 | MG019365 | Delibuti | New Zealand | ||
| C. turcopes cf. | PDD97513/CO1885 | KJ635235 | in ITS | /Turcopedes | New Zealand | ||
| C. ursus (type) | PDD70510/CO1075 | JX000381 | New Zealand | ||||
| C. variosimilis | TEB642-16 | MK358113 | MK358077 | MK340968 | /Varii | Russia | |
| C. velicopia cf. | CO611 | MK358114 | Subolivascentes | USA | |||
| C. vernicifer (type) | PDD88273/CO1654 | JX000401 | New Zealand | ||||
| C. verniciorum | JAC13232 | MK358115 | MK358078 | Verniciori | New Zealand | ||
| C. viscilaetus | JAC13736/PDD105941 | MH101575 | MH108413 | MH141053 | MH141023 | Limonii | New Zealand |
| PDD107734/CO2204 | KT875206 | in ITS | Limonii | New Zealand | |||
| PDD71010/CO812 | GU233378 | Limonii | New Zealand | ||||
| C. viscoviridis | PDD101840/CO2013 | JQ282171 | JQ282174 | New Zealand | |||
| C. vitreofulvus (type) | PDD97545/CO1920 | MH141036 | MH141018 | Marmorati | New Zealand | ||
| C. vitreofulvus cf. | PDD107727/CO2197 | KT875200 | in ITS | Marmorati | New Zealand | ||
| C. waiporianus | PDD107705/CO2175 | KT875191 | in ITS | Laeti | New Zealand | ||
| PDD95907/JAC11512 | MH101548 | MH108387 | MH141009 | Laeti | New Zealand | ||
| C. wallacei | JAC12076/PDD96316 | MH101552 | MH108391 | MH141030 | MH141025 | Subcastanelli | New Zealand |
| C. xenosma (type) | PDD73149/CO1182 | JX000389 | New Zealand | ||||
Phylogenetic reconstruction
The sequences were pre-checked and edited in MEGA 5.2 (Tamura et al. 2011). Multiple sequence alignments were performed separately on the individual gene regions using the online version of MAFFT v. 7 (Katoh & Standley 2013). We generated two datasets: a 2-loci (ITS+LSU) and a 4-loci (ITS+LSU+rpb1-rpb2) alignment. For the 2-loci dataset ITS and LSU sequences from 730 specimens were aligned separately using the E-INS-i algorithm (Katoh & Standley 2013), following Garnica et al. (2016). The alignments were manually corrected, trimmed and concatenated in SeaView 4 (Gouy et al. 2010). Preliminary analysis was run in PhyML 3.1 (Guindon & Gascuel 2003) using the following settings: GTR+I+G model of evolution, gamma distribution of 10 rate categories, and tree topology search as SPR.
Thereafter FastGap 1.2 (Borchsenius 2009) was used to code the phylogenetically informative insertion/deletion positions (indel) in both the ITS and LSU alignments following the simple indel coding algorithm (Simmons et al. 2001). After concatenating the nucleotide and binary data in SeaView 4, the partitioned alignment was submitted to maximum likelihood analysis using RAxML (Stamatakis 2014) as implemented in raxmlGUI 1.5.2 (Silvestro & Michalak 2012). The GTRGAMMA substitution model for the nucleotide partitions (ITS1+5.8S+ITS2+LSU) and the default setting for binary (indel) data was chosen. Rapid bootstrap analysis with 1 000 replicates was applied for testing branch support.
Based on the results of the 2-loci analyses, we selected representative sequences of species in each putative section to assemble a 4-loci dataset composed of 460 ITS, 417 LSU, 161 rpb1, and 87 rpb2 sequences. For the ITS and rpb1 loci we used the E-INS-i (Katoh & Standley 2013), for the LSU locus the G-INS-i (Katoh et al. 2005), and for the rpb2 locus the FFT-NS-i algorithms (Katoh et al. 2002), all under default settings. Referring to the 2-loci dataset (above), we used the same programs for manual inspection of the separate alignments as well as for concatenating the individual alignments and binary data. Six nucleotide (ITS1+5.8S+ITS2+LSU+rpb1+rpb2) and one binary (indel) partitions were defined in our supermatrix which was then submitted to raxmlGUI (Silvestro & Michalak 2012) with the same options as above. Alignments are available in TreeBase (S22220), newly generated sequences are deposited in GenBank (Table 1). The trees were in all cases edited and visualized in MEGA 7 (Tamura et al. 2013).
Section descriptions
Major morphological characters have been chosen for each new section. Lamellar colour always pertains to immature specimens. Odour, taste, and marginal lamellar elements are mentioned only when significant. The alkaline reaction was made with a 30 % NaOH (or KOH) solution. Unless otherwise specified, stipes are dry, and the hyphae are provided with clamp connections.
RESULTS
The final concatenated 2-loci data matrix comprises 730 sequences and 2 412 sites plus 1 324 binary characters, whereas the 4-loci data matrix comprises 460 sequences and 4 669 sites plus 1 118 binary characters. In total these datasets represent 601 Cortinarius species.
The resulting 4-loci and 2-loci phylograms (Fig. 1, 2) reveal a number of distinct clades, many of which exhibit a moderate (60–70 %; cf. Frøslev et al. 2005, Jeewon & Hyde 2016) to robust (80–100 %) bootstrap support values. The PhyML tree is not shown in this study, but its support values for sections and clades are mapped to the 2-loci phylogram (Fig. 2). Not unexpectedly we thereby recover a number of traditionally recognised and named sections and other supraspecific taxa. Other sections, preponderantly those with a bihemispherical or southern distribution, are described here as new. Some of these new taxa were anticipated in earlier works on the global phylogeny of the genus (Peintner et al. 2004, Garnica et al. 2005), and were sometimes given clade names that inform our section names. We identify 37 previously described sections, while 42 sections are here either described as new or based on taxa previously at a different rank. Monotypic sections (with the exception of Gigasperma) are not considered, even if typified by an included species. In addition, due to ongoing research into the taxonomy of several Cortinarius groups (including cases of insufficiently known taxa), 20 putative new sections are here discussed merely as named clades.
Fig. 1.
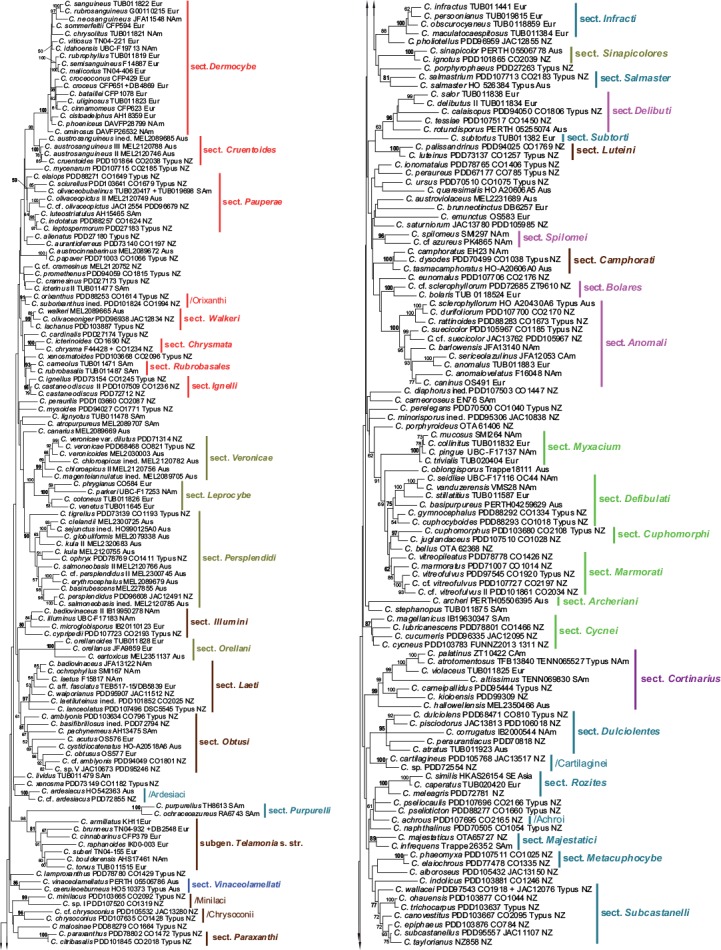

Maximum likelihood (RAxML) phylogenetic tree of a selection of the Cortinarius samples included in this study, depicting the supraspecific structure of the genus. The dataset of 460 samples consists of nrITS, nrLSU, rpb1, and rpb2 sequences with binary data from gap coding of ITS and LSU. Seven partitions are used: ITS1: 1–554, 5.8S: 555–705, ITS2: 706–1244, LSU: 1245–2355, rpb1: 2356–3901, rpb2: 3902–4670, BIN: 4671–6030. Vouchers and abbreviated geographical provenances are included in the labels. Assigned section or clade names are shown with gross morphological traits (i.e., belonging to the main categories of this study) indicated by colours (dark violet = sect. Cortinarius; red = dermocyboid sections; greenish yellow = leprocyboid sections; dark blue = Euphlegmacia + Calochroi; light blue = Pseudophelgmacia; pink = anomaloid groups; black = sect. Gigasperma; green = myxacioid sections; brown = telamonioid sections). RAxML bootstrap support values are shown only above 50 %.
Fig. 2.
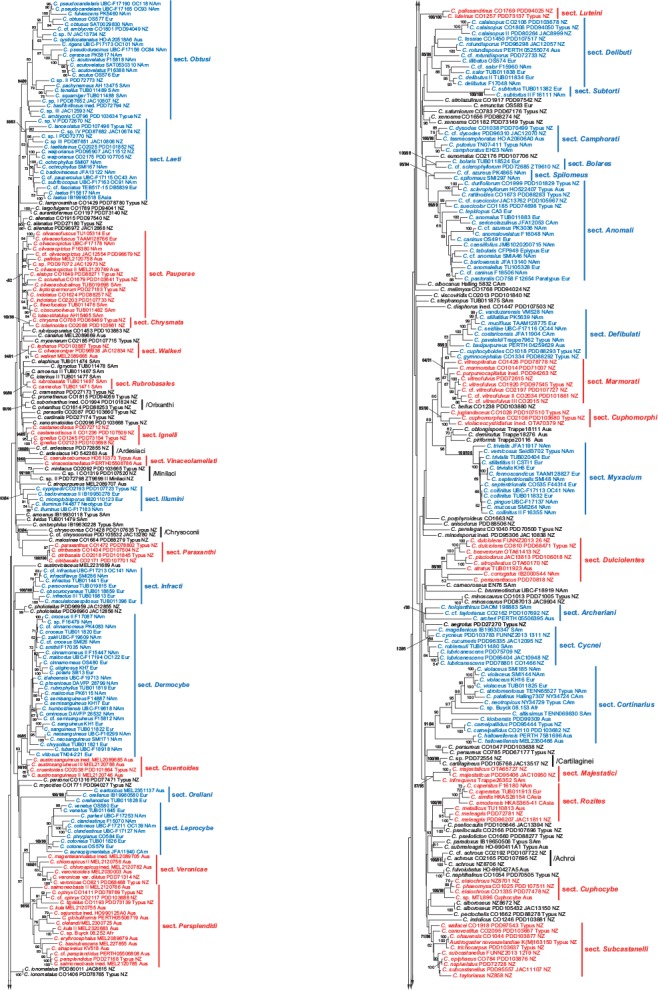
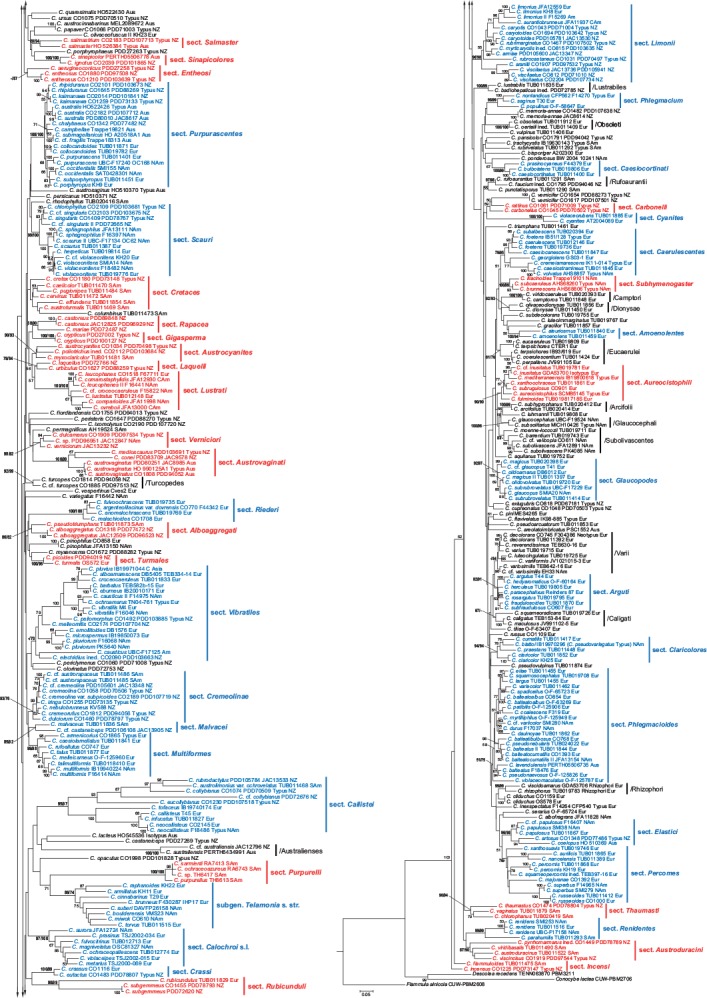
Maximum likelihood (RAxML) phylogenetic tree based on 730 Cortinarius samples and nrITS and nrLSU ribosomal genes with binary data from gap coding (BIN). Five partitions are used: ITS1: 1–610, 5.8S: 611–762, ITS2: 763–1334, LSU: 1335–2412, BIN: 2413–3736. Vouchers and abbreviated geographical provenances are included in the labels. Extant sections are marked blue, while new sections proposed in this study are marked red. Bootstrap support values are shown only above 50 %. RAxML support values, followed by the corresponding PhyML values, are shown in bold for sections and clades.
Morphological similarities among the species of a section are often obvious, even though clear synapomorphies are fairly rare. Especially in lineages with austral members, several phenotypic forms are sometimes present, while possessing other shared attributes that can be used to characterise the section. For example, a section may contain taxa with both agaricoid and sequestrate habits, but which all present a yellowish coloration and a viscid universal veil that may be regarded as sectional traits.
There are many singleton species in our phylograms, which our analysis could not associate with other taxa with any confidence (Fig. 1). Others appear to form loose but consistent associations with low bootstrap values; these are listed as Unsupported Groups in the context of a related section.
TAXONOMY
The sections are presented in a number of main groupings, largely based on the principal lineages recovered in the phylogeny of Fig. S2 in Garnica et al. (2016). The groupings also roughly correspond to subgenera of a traditional taxonomy (e.g., that of Brandrud et al. 1989, 1992, 1994, 1998, 2012) and are ordered accordingly. The following main groupings are used:
The type section (Cortinarius)
Dermocyboid sections
Leprocyboid sections
- Phlegmacioid sections
- 4.1. Euphlegmacia
- 4.2. Calochroi s.lat.
- 4.3. Pseudophlegmacia
Anomaloid sections
Gigasperma
Myxacioid sections
- Telamonioid sections
- 8.1. Subgenus Telamonia s.str.
- 8.2. Other telamonioid sections
For each section, the type is specified and the section or clade members are listed (epithets only, the name Cortinarius being subsumed). Our dataset comprises about 20 undescribed species whose formal protologues will be presented in future publications. Another 64 undescribed species are designated as ‘cf.’ or ‘C. sp.’.
In the species list the second column gives the known geographical provenance (see the abbreviations below). The third column specifies the source of genetic information, unless the species is sampled in both 2-loci and 4-loci trees (the normal case). Species that were only sampled in the 2-loci tree are marked 2L, and those that were sampled with at least one rpb sequence in the 4-loci tree are marked R. Species that were not sampled in our analysis, but were shown to belong to the same clade in Garnica et al. 2016 (based on ITS) are marked G2016. A few unpublished results from the ITS sequencing project of the DNA group of the European Cortinarius Association (Journées européennes du Cortinaire, JEC) were included and are marked JEC. Putative taxa that were not sampled in either study are listed in parentheses; these are taxa that have been described as being (likely) members of the section.
If at least two species were included in a section or clade, the ML bootstrap support (in %) is given. This refers to the 4-loci tree, unless specified otherwise. If the type of a new section is represented in GenBank (GB), this is also noted.
Notes on terminology
Refer to Fig. 3 and 4 for illustrations of some of these terms:
Fig. 3.

Phenotypic forms and habits of Cortinarius I. a. Agaricoid habit (C. violaceus); b. myxacioid habit (C. collinitus); c. dermocyboid habit (C. fervidus); d. leprocyboid habit (C. phrygianus); e. sequestrate habit 1 (C. beeverorum); f. sequestrate habit 2 (C. epiphaeus). — Photos K. Soop.
Fig. 4.

Phenotypic forms and habits of Cortinarius II. a. Phlegmacioid habit, stipitocarpic 1 (C. varius); b. phlegmacioid habit, stipitocarpic 2 (C. papulosus); c. phlegmacioid habit, pileocarpic (C. chlorophyllus); d. telamonioid habit (C. waiporianus); e. cuphocyboid habit (C. phaeomyxa); f. rozitoid habit (C. wallacei). — Photos K. Soop.
By agaricoid is meant the predominant habit of a Cortinarius basidiome with a pileus, stipe and distinct lamellae. The opposite term sequestrate, refers to a habit where the lamellae are replaced by a gleba, the pileus being more or less closed, and the stipe sometimes rudimentary.
Refer to the introductory text of headings 2, 3, 4.1, 5, 7, 8.1 for subgeneric adjectives (dermocyboid, telamonioid, etc.). By rozitoid is meant possessing a membranous partial veil (homologous with the cortina), like in the former genus Rozites. Cuphocyboid means lacking a cortina, like in the former genus Cuphocybe.
Pileocarpic and stipitocarpic refer to the development of a Cortinarius basidiome (Locquin 1953). The former implies an early expansion of the pileus, leaving an often marginate bulb on the stipe. In the latter case, the stipe develops earlier, leaving the stipe less bulbous, often clavate or cylindrical.
By boreal and austral we mean occurring in the Northern and Southern Hemisphere, respectively; bihemispherical implies both. These terms do not imply an ecological or climatological aspect.
The following geographical abbreviations are used: Eur (Europe), NAm/SAm/CAm (North/South/Central America), Aus (Australia), NZ (New Zealand).
1. Section Cortinarius (99 %)
Typus. C. violaceus (L.: Fr.) Gray.
| altissimus | Guyana | |
| atrotomentosus | NAm | R |
| carneipallidus | NZ | R |
| hallowellensis | Aus | R |
| kioloensis | Aus, NZ | R |
| neotropicus | CAm | 2L |
| palatinus | CAm | R |
| violaceus | Eur, NAm | R |
| (atroviolaceus | NZ, SE Asia) | |
| (hercynicus | Eur) | |
| (jenolanensis | Aus) | |
| (paraviolaceus | SE Asia) | |
| (subcalyptrosporus | NZ) |
Notes — The type section of the genus Cortinarius is widely distributed globally. The species are characterised by a dark blue to violet coloration overall, due to the (R)-β-dopa pigment, a dry velvety-granulose pileus, and lageniform cheilocystidia. See Harrower et al. (2015b).
The morphologically similar singletons C. atrolazulinus (New Zealand) and C. austroviolaceus (Australia) are placed in remote positions in our phylogeny, indicating that section characters are convergent. In addition, Moser (1986) described four southern species in the section (of which three from Malaysia, Borneo), but sequences of their holotypes have so far not been available.
2. Dermocyboid sections
This heading regroups taxa that correspond approximately to the Dermocybe lineage in Fig. S2 of Garnica et al. (2016). Basidiomata are usually small and slender, characterised by often brightly red/yellow/olive colours, due to anthraquinonic pigments, which also cause a positive (usually red) alkaline reaction in the tissues.
Section Dermocybe (Fr.) Gillot & Lucand (100 %)
Typus. C. cinnamomeus (L.: Fr.) Gray.
| aurantiobasis | NAm | G2016 |
| bataillei | Eur | R |
| cascadensis | NAm | G2016 |
| chrysolitus | NAm, Eur | R |
| cinnamomeoluteus | Eur, NAm | G2016 |
| cinnamomeus | Eur, NAm | R |
| cinnamomeus | II | 2L |
| cistoadelphus | Eur | R |
| croceoconus | Eur, NAm | R |
| croceus | Eur, NAm | R |
| croceus | II | 2L |
| fervidus | Eur | G2016 |
| harrisonii | NAm | G2016 |
| humboldtensis | NAm | 2L |
| huronensis | NAm | G2016 |
| idahoënsis | NAm | |
| malicorius | Eur, NAm | R |
| marylandensis | NAm | G2016 |
| neosanguineus | NAm | R |
| ominosus | Eur, NAm | |
| phoeniceus | Eur, NAm | |
| polaris | Eur | 2L |
| puniceus | Eur | G2016 |
| rubrophyllus | Eur | R |
| rubrosanguineus | Eur | R |
| sanguineus | Eur, NAm | R |
| semisanguineus | Eur, NAm | |
| sierraensis | NAm | G2016 |
| smithii | NAm | 2L |
| sommerfeltii | Eur | R |
| tillamookensis | NAm | G2016 |
| tubarius | Eur, NAm | 2L |
| uliginosus | Eur, NAm | R |
| vitiosus | Eur | R |
| zakii | NAm | |
| cf. cinnamomeus | NAm | 2L |
| cf. croceus | NAm | 2L |
| cf. semisanguineus | NAm | 2L |
| (cruentiphyllus | NAm, Eur) |
Notes — This large section is strictly boreal and consists of small to medium-sized fungi with a dry, felty/squamulose and non-hygrophanous pileus. The pigments are based on the octaketide pathway (Stensrud et al. 2014).
Cortinarius sect. Pauperae (M.M. Moser & E. Horak) Soop, comb. nov. (59 %, 82 % in PhyML tree)
Basionym. Dermocybe sect. Pauperae M.M. Moser & E. Horak, Beih. Nova Hedwigia 52: 500. 1975.
Typus. D. luteostriatula M.M. Moser & E. Horak.
MycoBank MB822986.
| elaiops | NZ | |
| indotatus | NZ | R |
| leptospermorum | NZ | |
| luteostriatulus (flavofucatus, obscurooliveus) | SAm | |
| olivaceobubalinus | SAm | R |
| olivaceobubalinus II | SAm | G2016 |
| olivaceofuscus | Eur | 2L |
| olivaceopictus | NAm | 2L |
| olivaceopictus II | Aus | R |
| sciurellus | NZ | R |
| cf. olivaceopictus | NZ | R |
| C. sp. | NZ | 2L |
| C. sp. II | Aus | 2L |
| (austronanceiensis | SAm) | |
| (cartagoënsis | CAm) | |
| (egmontianus | NZ) | |
| (nothovenetus | SAm) | |
| (olivaceoluteus | NAm) |
Notes — Pauperae contains dermocyboid species, typical for the Southern Hemisphere, of which only two are boreal. They present a yellow, olive, or citrinous coloration, with pigments of the skyrine and hypericine type (Stensrud et al. 2014). Unlike sect. Dermocybe, some members possess a glabrous or hygrophanous pileus. Three taxa (see C. luteostriatulus) are assumed conspecific with a 99.6 % similarity in the ITS-LSU region. The taxon C. sp. II was labelled ‘pallidus’ (Stefani et al. 2013), which would be a nom. illeg. (Cortinarius pallidus Peck 1889).
Cortinarius sect. Cruentoides Soop, sect. nov. (100 %)
Typus. C. cruentoides Soop, GB (ITS, LSU).
MycoBank MB822924.
| austrosanguineus ined. | Aus | R |
| austrosanguineus II | Aus | R |
| austrosanguineus III | Aus | R |
| cruentoides | NZ | R |
Basidiomata small, dry, reminiscent of the boreal subsect. Sanguinei. Pileus 10–30 mm diam, deep red, minutely granulose-fibrillose. Lamellae red. Stipe cylindrical, reddish. Veil dark red to purple red, rather copious. Context white to pinkish. Alkaline reaction blood red. Spores ellipsoid to subglobose, 6–8 × 4–5 μm, fairly coarsely verrucose. In Myrtaceae forests, New Zealand, Australia.
Notes — The section is sister to sect. Dermocybe, and might alternatively be considered part of the latter, which then becomes bihemispherical.
Cortinarius sect. Walkeri Soop, sect. nov. (99 %)
Typus. C. walkeri Cooke & Massee.
MycoBank MB822925.
| lachanus | NZ | |
| olivaceoniger | NZ | |
| walkeri (austrovenetus) | Aus | R |
Basidiomata small to medium-sized, dry to glutinous, yellowish to greenish. Pileus 10–60 mm diam, greenish, often with yellow or blue tints, minutely silky. Stipe cylindrical, pale green, flavescent or blushing. Lamellae citrinous, often blushing. Veil greenish, sparse. Context pale green to pale yellowish. Alkaline reaction blood red. Spores ellipsoid to amygdaloid, 8–10 × 4.5–6 μm, weakly verrucose. In Nothofagaceae and Myrtaceae forests, New Zealand, Australia.
Notes — The Patagonian sister taxon C. elaphinus deviates morphologically, and was described in subg. Telamonia. On the other hand, the morphologically similar C. alienatus from New Zealand appears as a singleton in our phylogeny.
Cortinarius sect. Chrysmata Soop, sect. nov. (100 %)
Typus. C. chrysma Soop, GB (ITS, LSU).
MycoBank MB822926.
| chrysma | NZ | R |
| icterinoides | NZ | R |
Basidiomata small, pileus dry, brilliantly yellow. Pileus 15–50 mm diam, yellow, finely fibrillose. Lamellae yellow. Stipe cylindrical to clavate, pale yellow. Veil dark yellow, sparse. Context yellow. Alkaline reaction blood red to vinaceous. Spores ellipsoid, 7–10 × 4.5–5.5 μm, weakly to moderately verrucose. In Nothofagaceae forests, New Zealand.
Cortinarius sect. Rubrobasales Soop & Dima, sect. nov. (93 %)
Typus. C. rubrobasalis M.M. Moser & E. Horak.
MycoBank MB822927.
| carneolus | SAm | |
| rubrobasalis | SAm | |
| teraturgus | SAm | G2016 |
Basidiomata telamonioid, small to medium sized, dry, red-brown. Pileus 30–70 mm diam, red-brown to date brown, minutely fibrillose. Lamellae pale brown to rusty yellow-brown. Stipe ± cylindrical, red-brown to pale reddish or yellowish. Veil incarnate to cinnabar red. Context white to red-brown. Alkaline reaction brownish to black. Spores ellipsoid, 8–10.5 × 4.5–6 μm, weakly verrucose. In Nothofagaceae forests, Patagonia.
Cortinarius sect.Ignelli Soop, sect. nov. (96 %)
Typus. C. ignellus Soop, GB (ITS, LSU).
MycoBank MB822928.
| castaneodiscus | NZ |
| castaneodiscus II | NZ |
| ignellus | NZ |
Basidiomata small, dry to viscid, yellow and reddish. Pileus 15–50 mm diam, yellow to orange-brown with a darker disc. Lamellae yellow-brown to olive yellow or orange. Stipe cylindrical, yellow with reddish veil remnants. Veil yellow to brick red. Context pale yellow to orange. Alkaline reaction red. Spores ovoid to subamygdaloid, 7–9.5 × 5–7 μm, moderately verrucose. In Nothofagaceae forests, New Zealand.
Clade /Orixanthi (91 %)
| orixanthus | NZ | R |
| suborixanthus ined. | NZ |
Notes — Basidiomata are medium-sized, viscid, coloration yellow to yellow-brown. In Nothofagaceae forests.
Unsupported group Icterinula
| alienatus | NZ | |
| amoenus II | SAm | 2L |
| cardinalis | NZ | |
| cramesinus | NZ | |
| icterinus II | SAm | |
| mycenarum | NZ | R |
| papaver (austrocinnabarinus) | NZ, Aus | R |
| peraurilis | NZ | R |
| promethenus | NZ | R |
| rubripurpuratus | NZ | 2L |
| xenosmatoides | NZ | |
| cf. cramesinus | Aus | 2L |
| (acutipapillatus [mastoideus] | Aus) | |
| (laetelamellatus | Aus) | |
| (vinicolor | NZ) |
Notes — The group has low support and is intermixed with several of the smaller sections treated above, and all the species in the list are singletons in the present study. Basidiomata are often brightly yellow and red, but C. xenosmatoides deviates by its drab telamonioid habit and lack of alkaline reaction. The Australian C. austrocinnabarinus is shown by our analysis to be a late synonym of C. papaver from New Zealand.
The type of Dermocybe sect. Icterinula, D. amoena, has been sequenced, but is poorly supported as a member of the group in our analysis. ITS sequences of the types of C. amoenus and C. icterinus are 99.8 % similar. Moreover, under a different interpretation, Garnica et al. (2003) place these two species in a separate clade, which was also recovered in our analysis (marked ‘II’ in the list). Due to these partial contradictions, Icterinula cannot be used as a section name in the present study.
3. Leprocyboid sections
In 1969 Moser described subg. Leprocybe, whose taxa are mainly characterised by yellow/brownish/greenish basidiomata whose context fluoresces in UV light. Many of the taxa have been shown to contain anthraquinonic pigments of the nonaketide pathway (Stensrud et al. 2014). Earlier studies (Peintner et al. 2004, Garnica et al. 2005, 2016) have shown that the subgenus is polyphyletic, while some of its sections are here recovered as clades. The South Pacific taxon C. canarius is basal to the leprocyboid clades, despite its original placement in the genus Dermocybe (cf. Stefani et al. 2013). Under the present heading we consider taxa that are approximately consistent with the concept of Leprocybe s. Moser. Only a few were sampled in Fig. S2 of Garnica et al. (2016).
Section Leprocybe (M.M. Moser) Melot (100 %)
Typus. C. cotoneus Fr.
| aureopigmentatus | CAm | 2L |
| clandestinus | NAm | 2L |
| cotoneus | Eur, NAm | R |
| flavifolius | NAm | G2016 |
| parkeri | NAm | |
| phrygianus | Eur | |
| venetus | Eur | |
| (cepistipes | Eur) | |
| (melanotus | Eur) | |
| (psittacinus | Eur) |
Notes — The boreal section is characterised by predominantly yellow and greenish olive basidiomata with a strong fluorescence, due to xanthone pigments. Section Persplendidi often forms a sister clade. See also Ammirati et al. (2007).
Cortinarius sect.Persplendidi Soop & Dima, sect. nov. (90 %)
Typus. C. persplendidus Gasparini, GB (ITS, LSU).
MycoBank MB822929.
Non subsect. Splendidi Bidaud, Moënne-Locc. & Reumaux.
| basirubescens (fuscoumbonatus) | Aus | R |
| clelandii | Aus | R |
| erythrocephalus | Aus | R |
| globuliformis | Aus | R |
| kula | Aus | R |
| kula II | Aus | R |
| melleilpileus ined. | Aus | Stefani et al. (2013) |
| ophryx | NZ | |
| persplendidus | NZ | R |
| salmoneobasis ined. | Aus | R |
| salmoneobasis II | Aus | R |
| sejunctus ined. | Aus | |
| sinapivelus | Aus | 2L |
| tigrellus | NZ | 2L |
| cf. persplendidus II | Aus | R |
| cf. ophryx | NZ | 2L |
Basidiomata agaricoid or sequestrate, dry, small to medium-sized, typically presenting a dark, tomentose pileus, a clavate stipe, and a yellow and strongly fluorescent context, recalling taxa in sect. Leprocybe. A few species dermocyboid (e.g., the blood-red C. kula). Anthraquinonic pigments based on the non-aketide pathway (Gill 1995). Pileus 10–60 mm diam, yellow, dark red-brown, dark orange-brown to umber or blackish, fibrillose/tomentose to granulose. Lamellae yellow, orange, or red. Stipe cylindrical to clavate or rudimentary, citrinous to saffron yellow or red. Veil red-brown to dark yellow, darkening, sparse to fairly copious. Context red to yellow. Alkaline reaction red to blackish brown, ± trivial in some species. Spores ovoid to subglobose, 8–11 × 5.5–8 μm, coarsely verrucose. In Nothofagaceae and Myrtaceae forest, New Zealand, Australia.
Notes — The section appears to be endemic for the South Pacific, but has so far not been recorded in Patagonia. The clade was named Splendidi in Garnica et al. (2005) and Stefani et al. (2013); however, to avoid connotation with Cortinarius splendidus Peck 1873, or with C. splendidus (E. Horak) K. Griffiths 1985, nom. illeg., we propose a new name here.
One of the members, C. cf. persplendidus II, has traditionally been named Dermocybe splendida in Australia, but forms a sister clade to the latter. Dermocybe splendida was described from New Zealand and later recombined as Cortinarius persplendidus (Gasparini 2006).
Cortinarius sect.Veronicae Soop, sect. nov. (99 %)
Typus. C. veronicae Soop, GB (ITS).
MycoBank MB822930.
| chloroapicus ined. | Aus | |
| chloroapicus II | Aus | |
| magenteiannulatus ined. | Aus | R |
| veronicae | NZ | R |
| veronicoides | Aus | R |
| (vinosipes | Aus) |
Basidiomata dermocyboid, dry, with cinnabar red to scarlet coloration, recalling the boreal C. cinnabarinus, fluorescence yellow. Pileus 20–50 mm diam, cinnabar red, tomentose to finely fibrillose. Lamellae red-orange to brick. Stipe cylindrical, pink to yellowish pink. Veil cinnabar-red or pink, fairly sparse to copious. Context pale yellow. Alkaline reaction blood red on pileus, bluish lilac on stipital veil. Spores subglobose, 5.5–7 × 4.5–5.5 μm, moderately verrucose. In Nothofagaceae forest, New Zealand, Australia.
Notes — The section is sister to sect. Leprocybe and might be considered part of the latter despite morphological differences (cf. Stefani et al. 2013).
Section Limonii Kühner & Romagn. ex Nezdojm. (99 %)
Typus. C. limonius (Fr.: Fr.) Fr.
| araniiti | NZ | 2L |
| armiae | NZ | |
| aurantiobrunneus | CAm | |
| caryotis | NZ | R |
| caryotoides | NZ | 2L |
| kroegeri | NAm | Liimatainen (2016) |
| limonius | Eur, NAm | |
| limonius II | Eur, NAm | 2L |
| myrticaryotis ined. | NZ | 2L |
| rubrimarginatus | NZ | |
| rubrocastaneus | NZ | 2L |
| viscilaetus | NZ | R |
Notes — This section, and the morphologically similar sect. Callistei, are recovered as well separated but closely related clades in our analyses. The taxa of both sections are characterised by vividly yellow and orange pigments, often with a positive alkaline reaction, but fluorescence is weak. Limonii has its core population in New Zealand with at least eight species, and no member has so far been reported from Australia. Two of them, Cortinarius rubrocastaneus and C. rubrimarginatus, possess remarkable chrysobasidia, a rare feature in Cortinarius. See further Soop et al. (2018).
Cortinarius sect.Callistei (Liimat., Niskanen & Ammirati) Soop, B. Oertel & Dima, stat. nov. (100 %)
Basionym. Cortinarius subg. Callistei Liimat., Niskanen & Ammirati, Index Fungorum 256: 2. 2015. IF551473.
Typus. C. callisteus (Fr.: Fr.) Fr., neotypus GB (ITS).
MycoBank MB823024.
| austrolimonius var. ochrovelatus | SAm | R |
| callisteus | Eur, NAm | |
| collybianus | NZ | R |
| controversus | Aus | R |
| eucollybianus | NZ | |
| infucatus | Eur, NAm | R |
| neocallisteus | NAm, Eur | |
| rubrodactylus | NZ | |
| tophaceus | Eur, NAm | 2L |
| cf. collybianus | NZ | 2L |
Notes — Cortinarius austrolimonius was described in the South American subg. Cystogenes, characterised by the presence of remarkable cheilocystidia. But the type (C. formosus) of the subgenus has not been sequenced, and remaining species in the subgenus do not exhibit this character state. Garnica et al. (2003) grouped C. austrolimonius var. ochrovelatus with C. pugionipes and C. cervinus, also from South America, but this affinity is not recovered in our analysis. See further Niskanen et al. (2016).
Section Orellani M.M. Moser (100 %)
Typus. C. orellanus Fr.
| eartoxicus | Aus | 2L |
| orellanoides | Eur, NAm | R |
| orellanus | Eur, NAm | R |
| (catarracticus | Aus) | |
| (fluorescens | SAm) |
Notes — This bihemispherical section was placed by Moser (1969) in subg. Leprocybe, and later promoted to subgeneric rank by Gasparini (2004). It is characterised by yellow and orange pigments and is unique in Cortinarius by containing appreciable quantities of the lethal toxin orellanine.
Cortinarius sect.Sinapicolores (Gasparini) Soop, stat. nov. (100 %)
Basionym. Cortinarius subser. Sinapicolores Gasparini, New Zealand J. Bot. 45: 228. 2007.
Typus. C. sinapicolor Cleland.
MycoBank MB822989.
| sinapicolor | Aus |
| ignotus | NZ |
| (wirrabara | Aus) |
Notes — This small southern clade consists of medium-sized, glutinous cortinars of a golden-yellow colour that react strongly with alkaline solutions. The type of the section is also part of sect. Pyromyxa M.M. Moser, a name we cannot use, since no sequence of its type, C. pyromyxa, is available.
Cortinarius sect.Rubicunduli Soop, B. Oertel & Dima, sect. nov. (100 %)
C. stirps rubicundulus M.M. Moser in Singer (1986).
Typus. C. rubicundulus (Rea) A. Pearson.
MycoBank MB822931
| paurigarhwalensis ined. | C Asia | Fungal Diversity Notes (in prep.) |
| rubicundulus | Eur | R |
| subgemmeus | NZ |
Basidiomata agaricoid, medium-sized, dry to viscid, coloration yellowish with a tendency to darken on bruising, fluorescence weak. Pileus 15–70 mm diam, pale yellow to yellow-brown with orange-red to dark brown spots and zones, matt, mottled with reddish to pale ochraceous fibrils, blushing to dark orange. Lamellae greyish yellow to pale tan. Stipe cylindrical to clavate, white to greyish yellow, blushing or flavescent. Veil, pale grey to pale ochraceous, blushing and darkening to red or orange, rather copious to sparse. Context pale yellow to tan, ± flavescent when cut and bruised. Alkaline reaction insignificant. Spores ellipsoid to subfusoid, 6–9 × 3.8–5 μm, weakly verrucose. Cheilo- and pleurocystidia prominent, cylindrical or capitate. In Picea, Nothofagaceae, and Quercus leucotrichophora forests, Europe, New Zealand, Asia, respectively.
Notes — These species are recovered on very long branches in a basal position of the phylogeny. The section is a strongly supported sister to Crassi, indicating an affinity consistent with some shared morphological characters, such as the cheilocystidia and a soft context.
Cortinarius sect.Incensi Soop, sect. nov. (79 %)
Typus. C. incensus Soop, GB (ITS, LSU).
MycoBank MB822932.
| flammuloides | SAm |
| incensus | NZ |
Basidiomata agaricoid, medium-sized, yellowish. Pileus 15–80 mm diam, slightly viscid, saturated to pale yellowish, disk often more orange with brownish stains or squamules. Lamellae whitish to grey-yellow. Stipe cylindrical, often slightly bulbous/clavate, pale yellow to orange, staining brownish. Veil yellow to orange-brown, fairly copious. Context white, often flushing yellow with age or on manipulation. Odour ± spicy. Alkaline reaction yellow to red-brown or red, fluorescence greenish yellow. Spores ellipsoid to subcitriform, 9–12 × 5–6.5 μm, moderately verrucose. In Nothofagaceae forest, South Pacific.
Notes — Moser in Singer (1986) assigned the Patagonian member to stirps Flammuloides in subg. Phlegmacium.
4. Phlegmacioid sections
4.1. Euphlegmacia
Under this provisional name we regroup most of the taxa that were included in Phlegmacioid lineages I and II in Garnica et al. (2016: Fig. S2). They are traditionally and morphologically assigned to subg. Phlegmacium, characterised by a viscid pileus, a dry stipe, relatively crowded lamellae, and a more or less robust habit, even though a fair number of exceptions do occur. The Calochroi complex is discussed in the next subchapter.
Section Phlegmacium (Fr.) Gillot & Lucand (99 %)
Typus. C. saginus (Fr.: Fr.) Fr.
| norrlandicus | Eur | |
| populinus | Eur | |
| saginus | Eur | R |
Notes — The section is boreal and includes the type of the traditional subg. Phlegmacium. Basidiomata are stipitocarpic and present a yellow to brownish veil. But C. triumphans, often assigned here, occupies an isolated position.
Section Scauri (Fr.) Henn. (100 %)
Typus. C. scaurus (Fr.: Fr.) Fr., neotypus GB (ITS).
| chlorophyllus | NZ | |
| fuligineofolius | NAm | G2016 |
| herpeticus | Eur | 2L |
| montanus | NAm | 2L |
| scaurus | Eur, NAm | R |
| scaurus II | Eur | 2L |
| singularis | NZ | R |
| sphagnophilus | Eur, NAm | 2L |
| violaceonitens | Eur | |
| virentophyllus | NAm | G2016 |
| cf. singularis | NZ | 2L |
| cf. singularis II | NZ | 2L |
| cf. violaceonitens | Eur | 2L |
Notes — This section is bihemispherical and sister to sect. Purpurascentes. The morphological and chemical plasticity within the section is remarkably low. The species are characterised by a pileocarpic development, a viscid pileus, often with an olive or greenish tint, and a positive iodine-based reaction (Garnica et al. 2005).
Section Purpurascentes M.M. Moser (100 %)
Typus. C. purpurascens Fr., neotypus GB (ITS).
| argyrionus | Aus | G2016 |
| australis | Aus, NZ | |
| caesibulga | Aus | G2016 |
| campbellae | Aus | 2L |
| chalybaeus | NZ | R |
| cinereoroseolus | Aus | G2016 |
| collocandoides | Eur | 2L |
| kaimanawa | NZ | R |
| occidentalis (mutabilis) | NAm, Eur | 2L |
| porphyropus | NAm, Eur | |
| purpurascens | NAm, Eur | R |
| rhipiduranus | NZ | |
| submagellanicus | Aus | |
| subporphyropus (mendax) | Eur | 2L |
| subpurpurascens | NAm, Eur | G2016 |
| cf. fragilis | Aus | 2L |
Notes — This section is bihemispherical. Like in Scauri (above) the basidiomata present a positive iodine-based reaction, though most deviate by a stipitocarpic habit, and four of the Australian species are sequestrate. In addition, parts of the basi-diomata typically darken with a violet tinge on bruising (Saar et al. 2014). Cortinarius cf. fragilis appears to be a sequestrate form of a morphospecies that includes the agaricoid C. submagellanicus. The morphologically deviating South Pacific singleton C. persicanus is a sister.
Section Multiformes (Rob. Henry) Moënne-Locc. & Reumaux (100 %)
Typus. C. multiformis Fr., neotypus GB (ITS).
| armenicorius | Eur, Asia | 2L |
| caesiolamellatus | NAm, Eur | 2L |
| caesiophylloides | Eur, NAm | G2016 |
| frondosomultiformis | Eur | JEC |
| melleicarneus | Eur | 2L |
| multiformis | NAm, Eur | |
| pallidirimosus | NAm, Eur | G2016 |
| rufoallutus | Eur | |
| talimultiformis | Eur | |
| talus | Eur, NAm | R |
Notes — The section appears to be endemic to the Northern Hemisphere. See further Liimatainen et al. (2014) and Brandrud et al. (2014).
Section Cremeolinae Soop (93 %)
Typus. C. cremeolina Soop, GB (ITS, LSU).
| cremeolina | NZ | |
| cremeolina var. subpicoides | NZ | |
| cremeorufus | NZ | |
| dulciorum | NZ | |
| iringa | NZ | |
| nebulobrunneus | Aus | 2L |
| cf. austrorapaceus | SAm | |
| cf. cremeolina | NZ | 2L |
| cf. cremeolina II | NZ | 2L |
Notes — This is an austral section morphologically similar to the sister sect. Multiformes. The Australian taxon, however, is sequestrate. See further Soop (2016).
Section Claricolores Moënne-Locc. & Reumaux (95 %)
Typus. C. claricolor Fr., neotypus GB (ITS).
| blattoi (pseudovariegatus) | Eur, NAm | 2L |
| claricolor | NAm, Eur | R |
| cumatilis | Eur | R |
| praestans | Eur | |
| rex-claricolorum | Eur | G2016 |
Notes — See further Brandrud et al. (2013).
Clade /Rhizophori (100 %)
| rhizophorus | Eur | R |
| viscidoamarus | Eur |
Notes — Taxa in this boreal clade possess a slightly bulbous stipe and yellowish tints.
Section Elastici (Fr.) Henn. (100 %)
Typus. C. papulosus Fr., neotypus GB (ITS).
| artosus | NZ | |
| castaneicolor | NAm | G2016 |
| coelopus | Aus | |
| luteobrunnescens | Eur, NAm | G2016 |
| ochraceobrunneus | Eur | G2016 |
| papulosus | Eur, NAm | R |
| cf. papulosus | Eur, NAm | 2L |
Notes — Taxa in this bihemispherical section possess clavate stipes and often have a characteristic grassy odour. The section is strongly supported as sister to the following one, but is kept segregated from it due to morphological differences.
Section Percomes (Moënne-Locc. & Reumaux) Melot (77 %)
Typus. C. percomis Fr., neotypus GB (ITS).
| aurilicis | Eur | R |
| cephalixoides | NAm | G2016 |
| citrinifolius | NAm | G2016 |
| nanceiensis | Eur | R |
| pallidopercomis ined. | Eur | G2016 |
| percomis | Eur | R |
| russeoides | Eur | 2L |
| squameopercomis ined. | Eur | 2L |
| stjernegaardii | Eur | G2016 |
| superbus | NAm | |
| xanthosuavis | Eur | |
| (mussivus | Eur) |
Notes — This boreal section consists of yellowish taxa, often with a citrinous or olive tint, and mostly a stipitocarpic habit. Many have characteristic odours.
Section Caesiocortinati Frøslev & T.S. Jeppesen (100 %)
Paronym. C. subsect. Caesiocortinati Brandrud & Melot.
Typus. C. caesiocortinatus Jul. Schäff.
| bulbolatens (turbinatorum) | Eur | |
| caesiocortinatus | Eur | R |
| prasinocyaneus | Eur | R |
| cf. caesiocortinatus | Asia | JEC |
Notes — Basidiomata are large with an irregularly bulbous stipe. The spores are subglobose and strongly verrucose. The North American C. ponderosus is closely related to the section, but not morphologically similar.
Section Phlegmacioides (Fr.: Fr.) Brandrud, H. Lindstr. & Melot (80 %)
Typus. C. variecolor (Pers.: Fr.) Fr., neotypus GB (ITS).
| areni-silvae | Eur | R |
| balteatialutaceus | Eur | G2016 |
| balteatibulbosus | Eur | R |
| balteatoalbus | Eur | |
| balteatocumatilis | NAm, Eur | R |
| balteatocumatilis II | NAm | G2016 |
| balteatus | NAm, Eur | |
| balteatus II | NAm | 2L |
| brunneiaurantiaus | Eur | G2016 |
| brunneolividus | Eur | G2016 |
| caesiocolor | Eur | G2016 |
| coalescens | Eur | 2L |
| daulnoyae (chromataphilus) | Eur | R |
| durus | Eur | |
| eliae (lividoviolaceus) | Eur | 2L |
| largus | Eur | 2L |
| lavendulensis | Aus | |
| myrtilliphilus | Eur | 2L |
| patibilis | Eur | |
| pseudodaulnoyae (squamosocephalus) | Eur | 2L |
| pseudonaevosus (vacciniophilus) | Eur | 2L |
| pseudonebularis | Eur | 2L |
| sobrius | Eur | G2016 |
| spadicellus | Eur | |
| variecolor | NAm, Eur | R |
| violaceomaculatus | Eur | R |
| cf. variecolor | NAm | 2L |
Notes — Apart from one Australian species, the section is boreal. It consists of stipitocarpic taxa of Phlegmacium habit, though the pileus is sometimes almost dry. The core of the section consists of subsect. Variecolores and Balteati (Brandrud 1998).
Clade /Varii (86 %)
| decolorans | Eur | |
| luteocingulatus | Eur | 2L |
| reverendissimus | Eur | R |
| variiformis | Eur | 2L |
| variosimilis | NAm, Eur | R |
| varius | Eur | |
| cf. variosimilis | NAm |
Notes — Basidiomata are stipitocarpic with an ochraceous pileus, violaceous lamellae, and a white to yellow veil. In coniferous and broad-leaf forests.
Cortinarius decolorans (Pers.) Fr.
Neotypus. Sweden, Gotland, Tjaukle Änge, with Picea, K. Soop CO745, herb. S F304386, GenBank KJ421062 (ITS+LSU), hic designatus.
MycoBank MBT378826.
Notes — This species was labelled ‘C. varius II’ in Garnica et al. (2016). Basidiomata resemble C. varius, but (almost) lack violet hues. They match Fries’ taxon well (Fries 1821, 1851); we hence propose to neotypify his epithet for the present taxon, even though the name has not often been used in modern literature.
Clade /Obsoleti (100 %)
| obsoletus | Eur | R |
| oertelii ined. | Eur | R |
Notes — Basidiomata are stipitocarpic with an ochraceous pileus, violaceous lamellae, and a white veil. In broad-leaf forests.
Section Amoenolentes Brandrud & Melot (99 %)
Typus. C. amoenolens Rob. Henry.
| aleuriosmus | Eur | R |
| amoenolens (anserinus s. auct.) | Eur | |
| griseocoeruleus | Eur | G2016 |
Notes — A boreal section of pileocarpic and odorous fungi with violaceous lamellae. Clade /Dionysae may be considered part of the section, despite deviating in several characters. See further Fernández-Brime et al. (2014) and Liimatainen et al. (2014).
Clade /Dionysae (96 %)
| boreidionysae | Eur | JEC |
| dionysae | NAm, Eur | R |
| dionysae II | Eur | G2016 |
| mahiquesii | Eur | G2016 |
| olivaceodionysae | Eur | |
| palazonianus | Eur | G2016 |
Notes — Taxa are pileocarpic with a greyish or olivaceous pileus, violaceous lamellae, and often a farinaceous odour.
Clade /Camptori (100 %)
| calyptrodermus | NAm | G2016 |
| camptoros | Eur | |
| velicopia | NAm | JEC |
| viridicoeruleus (lepistoides) | Eur | R |
Notes — Basidiomata are pileocarpic, medium-sized, with a typically viscid and hygrophanous cutis. In broad-leaf forests.
Cortinarius sect.Turmales Soop, B. Oertel & Dima, sect. nov. (100 %)
Typus. C. turmalis Fr., neotypus GB (ITS).
MycoBank MB822933.
| picoides | NZ | R |
| turmalis (corrugis) | Eur, NAm | R |
Basidiomata stipitocarpic, medium-sized, often caespitose. Pileus 30–100 mm diam, yellow-brown to dark brown, viscid, finely fibrillose. Lamellae greyish white, crowded. Stipe cylindrical to tapering and radicant, white, silky fibrillose, often staining brownish or violaceous. Veil white, rather sparse. Alkaline reaction insignificant. Spores fusoid to amygdaloid, 6.5–9 × 3.3–4.5 μm, weakly verrucose. In Picea and Nothofagaceae forests, Europe, North America, South Pacific.
Cortinarius sect.Aureocistophili Fern.-Brime ex Soop, B. Oertel & Dima, sect. nov. (87 %)
Typus. C. aureocistophilus Vila, Contu & Llimona, GB (ITS, LSU).
MycoBank MB822934.
| aureocistophilus | Eur | |
| fulminoides | Eur | |
| inusitatus | Eur | 2L |
| kytoevuorii | Eur | G2016 |
| mediterranensis | Eur | |
| subrugulosus | Eur | |
| xanthoochraceus | Eur | R |
| cf. inusitatus | Eur | G2016 |
Basidiomata pileocarpic or stipitocarpic, medium-sized to large, evoking species of both sect. Multiformes and sect. Glaucopodes. Pileus 30–120 mm diam, viscid, yellow to orange or ochraceous, rarely violaceous when young, finely to coarsely white fibrillose, often with veil remnants near margin. Lamellae greyish white, rarely with a pink tinge. Stipe cylindrical with a rounded or marginate bulb, white, often flushing yellow with age. Veil white to yellowish, rarely with a blue tinge, sparse to rather copious. Context white, often flushing yellow with age or manipulation. Alkaline reaction reddish to brownish, or insignificant. Spores ellipsoid to amygdaloid, 7.5–11 × 4.5–6 μm, moderately verrucose. Mainly in Picea, Abies and Quercus forests, Europe.
Notes — See further Fernández-Brime et al. (2014).
Section Riederi (Brandrud & Melot) Brandrud, Dima, Niskanen & Liimat. (100 %)
Typus. C. riederi (Weinm.) Fr., neotypus Melot (1986) (ITS in Brandrud et al. 2018).
| anomaloochrascens | Eur | R |
| argenteolilacinus | Eur | G2016 |
| var. dovrensis | Eur | 2L |
| burlinghamiae | NAm | JEC |
| fulvoochrascens | NAm, Eur | R |
| glaucocyanopus | Eur | JEC |
| malachioides | Eur | |
| pallidoriederi | Eur | JEC |
| parksianus | NAm | JEC |
| riederi (pseudoarquatus) | NAm, Eur | G2016 |
Notes — A boreal section of robust taxa evoking members of sect. Glaucopodes. See Brandrud et al. (2018).
Section Glaucopodes (Konrad & Maubl.) Moënne-Locc. & Reumaux (99 %)
Typus. C. glaucopus Fr., neotypus GB (ITS).
| alticaudus | Eur, NAm | G2016 |
| cistoglaucopus | Eur | G2016 |
| glaucopus | NAm, Eur | |
| glaucopus II | Eur | G2016 |
| glaucopus III | Eur | G2016 |
| magicus | Eur | R |
| magicus II | Eur | 2L |
| olidoamarus (misermontii, van-campiae) | Eur | R |
| olidovolvatus | Eur | |
| pansa | Eur | G2016 |
| perstrenuus (subaccedens) | Eur | G2016 |
| subfoetens | NAm | G2016 |
| subrubrovelatus | Eur | R |
| tirolianus | Eur | G2016 |
| cf. glaucopus | Eur | 2L |
Notes — A boreal section of pileocarpic fungi, often with violet lamellae and a fibrillose cutis.
Clade /Arcifolii (94 %)
| arcifolius | Eur | R |
| subhygrophanus | Eur | R |
| cf. arcifolius | Eur | JEC |
Notes — Basidiomata are pileocarpic, medium-sized, with a pale to dark ochraceous coloration, often with an olivaceous tint, veil yellowish. In Fagaceae forests.
Clade /Glaucocephali (99 %)
| glaucocephalus | NAm |
| subsolitarius | NAm |
Notes — Basidiomata are pileocarpic, medium-sized, dark greenish, lamellae blue. In coniferous forests.
Section Arguti (Brandrud & Melot) Liimat., Ammirati, Niskanen, Dima & C. Cripps (82 %)
Typus. C. argutus Fr.
| argutus | NAm, Eur | |
| fraudulosus | NAm, Eur | JEC |
| fraudulosoides | Eur, NAm | |
| hedyaromaticus | NAm, Eur | 2L |
| herculeus | Eur | R |
| paracephalixus | Eur | |
| patrickensis | NAm, Eur | G2016 |
| rioussetiae | Eur | G2016 |
| rosargutus | Eur | 2L |
| subfraudulosus | Eur |
Notes — A boreal section of stipitocarpic fungi, whose stipe often tapers towards the base coupled with a caespitose growth. Colours are mostly pale, the lamellae conspicuously crowded, and the pileus is only slightly viscid or even dry. The European Cortinarius pseudovulpinus is morphologically similar but not closely related.
Clade /Caligati (67 %)
| caligatus | Eur | |
| maculosus | Eur | |
| squameoradicans | Eur | R |
Notes — Closely related to the morphologically similar sect. Arguti. Basidiomata are stipitocarpic, with the stipe tapering towards the base and a copious veil. Growth caespitose, in Fagaceae forests.
Section Caerulescentes Rob. Henry ex Moënne-Locc. & Reumaux (90 %)
Typus. C. caerulescens (Schaeff.: Fr.) Fr., neotypus GB (ITS).
| albescens | NAm, Eur | G2016 |
| caerulescens | NAm, Eur | |
| caesiocanescens | Eur | R |
| caesiostramineus | Eur | R |
| cremeiamarescens | NAm, Eur | 2L |
| foetens (aurantiobasalis) | Eur | |
| georgiolens | Eur | R |
| subalbescens | Eur | R |
| volvatus | NAm, Eur | 2L |
Notes — A boreal section of pileocarpic fungi, most of which display violaceous colours.
Clade /Eucaerulei (96 %)
| aurescens | NAm | JEC |
| caerulescentium | Eur | R |
| eucaeruleus | Eur | R |
| perpallens | Eur | |
| terpsichores | NAm, Eur |
Notes — Basidiomata are medium-sized, pileocarpic with violaceous hues, resembling species in the sister sect. Caerulescentes. In broad-leaf forests.
Clade /Subolivascentes (89 %)
| atrochalybaeus | NAm, Eur | G2016 |
| barrentium (tauri) | NAm, Eur | R |
| moënne-loccozii | Eur | |
| subolivascens | NAm | |
| cf. atrochalybaeus | NAm | JEC |
| cf. velicopia | NAm | 2L |
Notes — Basidiomata medium-sized, pileocarpic, coloration brown to violaceous. In coniferous and broad-leaf forests.
Cortinarius sect.Vinaceolamellati Soop & Gasparini, sect. nov. (86 %)
Typus. C. vinaceolamellatus Cleland.
MycoBank MB822935.
| caeruleoëburneus | Aus |
| vinaceolamellatus | Aus |
Basidiomata medium-sized, stipitocarpic. Pileus 30–60 mm diam, viscid, pale violaceous, brunnescent, finely fibrillose. Lamellae lilac-violet. Stipe clavate, white to pale violet. Veil white to pale violet, sparse to rather copious. Context white to pale brownish, marbled violet. Alkaline reaction insignificant. Spores ellipsoid to ovoid, 7.5–10.5 × 4.5–6.5 μm, moderately verrucose. Cheilocystidia prominent in one species. In Myrtaceae forests, Australia.
Cortinarius sect.Alboaggregati Soop, sect. nov. (100 %)
Typus. C. alboaggregatus Soop, GB (ITS).
MycoBank MB822936.
| alboaggregatus | NZ | R |
| pseudotriumphans | SAm | R |
Basidiomata medium-sized to large, stipitocarpic. Pileus 35–75 mm diam, viscid, white to yellow-brown, somewhat brunnescent on disk, finely fibrillose, margin involute. Lamellae white, crowded. Stipe tapering downwards or fusoid, rooted, white with rather thick, white girdles, often peronate. Veil white, copious. Context white. Alkaline reaction orange-brown or insignificant. Spores fusoid-amygdaloid, 10.5–13.5 × 5.5–7.5 μm, moderately verrucose. In Nothofagaceae forests, New Zealand and South America.
Cortinarius sect.Cretaces Soop & Dima, sect. nov. (57 %, 90 % in PhyML tree)
Typus. C. cretax Soop, GB (ITS).
MycoBank MB822937.
| austroturmalis | SAm | |
| caelicolor | SAm | |
| cervinus | SAm | |
| cretax | NZ | |
| effundens | SAm | |
| lacteus | Aus | 2L |
| pugionipes | SAm | 2L |
| (xiphidipus | SAm) |
Basidiomata medium-sized to large, stipitocarpic, often caespitose. Pileus 25–120 mm diam, viscid, white to yellow-brown, occasionally flavescent or brunnescent on disk, glabrous to finely fibrillose. Lamellae white to pale grey-brown, crowded. Stipe tapering downwards, rooted, white, ± glabrous. Veil white, sparse. Context white. Odour weak or like bitter almonds. Alkaline reaction insignificant. Spores fusoid-amygdaloid, 6–10 × 3–5.5 μm, weakly verrucose. In Nothofagaceae forests, South Pacific.
Notes — This predominantly Patagonian section is part of stirps Xiphidipus (see Moser & Horak 1975), but as no sequence of the paronymous species C. xiphidipus is available, we cannot use the name as the base of a new combination. The fungi resemble those of sections Arguti and Alboaggregati, which are genetically remote.
Clade /Turcopedes (99 %)
| turcopes | NZ |
| cf. turcopes | NZ |
Notes — A small austral clade of stipitocarpic fungi with a blue to blue-green coloration. In Nothofagaceae forests.
4.2. Calochroi s.lat.
This large monophyletic group of over 80 species appears to be endemic for the Northern Hemisphere. Basidiomata generally present a pileocarpic habit with a wide stipital bulb. Many of the species are rare and most grow exclusively on calcareous soil. Their hosts belong to a range of broad-leaf and coniferous genera, but Quercus is a dominant partner, especially in the southern parts of the region.
The phylogeny reveals a number of closely related clades, many of which are recovered as traditional sections. These are not further discussed in this study, having been extensively documented in several dedicated efforts (Frøslev et al. 2006a, b, Ortega et al. 2008, Garnica et al. 2009, 2011). They are represented in Garnica et al. (2016: Fig. S2) by Phlegmacioid clade III.
4.3 Pseudophlegmacia
Under this provisional name we consider taxa that in various respects (habit, viscidity, hygrophanity, etc.) deviate from typical Phlegmacium, and/or have sometimes been assigned to other genera or subgenera. Only a few were sampled in Fig. S2 of Garnica et al. (2016).
Cortinarius sect.Lustrati Ammirati ex Soop, B. Oertel & Dima, sect. nov. (100 %)
Typus. C. lustratus Fr.
MycoBank MB822938
| comparioides | CAm | 2L |
| leucophanes (comarostaphylides) | Eur, NAm, CAm | |
| leucophanes II | NAm | 2L |
| lustratus | Eur | R |
| oregonensis | CAm | G2016 |
| ovreboii | CAm |
Basidiomata medium-sized to small, stipitocarpic, pale. Pileus 25–70 mm diam, viscid, ivory to creamy-yellow or pale violet, disk yellowish, glabrous to silky. Lamellae greyish, sometimes with a rosy or violet tinge, crowded. Stipe clavate to cylindrical with a small bulb, silky white, often ± violaceous at apex. Veil white to pale violet, sparse to fairly copious. Context white, occasionally marbled violet. Odour none or farinaceous, sometimes fruity. Alkaline reaction insignificant. Spores 5.5–8 × 3.5–4.5 μm, ellipsoid, weakly verrucose. In Pinus, Fagaceae, and Comarostaphylis forests, Europe, North/Central America.
Notes — The boreal section forms a well-supported clade with two sister sections in the South Pacific, Laquelli and Austrocyanites. See further Ammirati et al. (2007).
Cortinarius sect.Laquelli Soop, sect. nov. (80 %)
Typus. C. laquellus Soop, GB (ITS).
MycoBank MB822939.
| laquellus | NZ |
| urbiculus | NZ |
Basidiomata small, stipitocarpic, coloration pale, recalling those of sect. Lustrati. Pileus 7–35 mm diam, dry to viscid, matt with a white, velvety or pruinose coating, finely fibrillose. Lamellae pale violet to greyish violet, medium crowded. Stipe cylindrical to clavate, silvery white with a faint violaceous tint. Veil white, fairly copious. Context white, young marbled violet or entirely bright violet-lilac, odour faint, ± like wax candles, taste ± bitter. Alkaline reaction insignificant. Spores ellipsoid to subamygdaloid, 6.5–8.5 × 3.8–5 μm, weakly to moderately verrucose. In Nothofagaceae forests, New Zealand.
Cortinarius sect.Thaumasti Soop, sect. nov. (93 %)
Typus. C. thaumastus Soop, GB (ITS, LSU).
MycoBank MB822965.
| chlorophanus | SAm | R |
| thaumastus | NZ | |
| vaginatus | SAm | R |
| (coleopus | SAm) |
Basidiomata small, with a peronate or volvate stipe. Pileus 15–50 mm diam, dry to slightly viscid, hygrophanous, olive-green, yellow, or apricot-brown to red-orange, finely granulose to fibrillose, with white to ochraceous tufts. Lamellae pale greyish to ochraceous, fairly crowded. Stipe with a ± marginate bulb, white to pale yellow, orange, or olivaceous. Veil white to ochraceous, fairly copious, on stipe fibrillose to peronate with thick tufts near base, sometimes forming a membranous volva or collar. Context greyish ochraceous to olivaceous. Alkaline reaction orange to red-brown in context, red to red-brown on cutis. Spores citriform to amygdaloid, 8–11 × 3.5–5 μm, up to 7 μm wide in one species, weakly verrucose. In Nothofagaceae forests, New Zealand and Patagonia.
Notes — Several taxa in this austral section are members of Cortinarius subsect. Coleopodes (see Moser & Horak 1975). However, since no sequence of the type C. coleopus is available, we cannot use Coleopodes as a basionym.
Clade /Rufoaurantii (79 %)
| faucium ined. | NZ |
| rufoaurantius | SAm |
Notes — A small austral clade of phlegmacioid/telamonioid taxa. Basidiomata are small to medium-sized, stipitocarpic; the coloration is yellow-brown to orange-brown. In Nothofagaceae forests.
Cortinarius rufoaurantius Soop, nom. nov.
Basionym: Cortinarius aurantiorufus Garnica, in Garnica et al., Mycologia 94 (1):136. 2002, nom. illeg.
MycoBank MB823022.
Notes — The name C. aurantiorufus Bidaud is in use (Bidaud et al. 2001).
Cortinarius sect.Dulciolentes Soop, sect. nov. (95 %)
Typus. Cuphocybe melliolens Soop, GB (ITS).
MycoBank MB822940.
| atratus (P. luteum) | Aus, NZ | R |
| atropileatus | NZ | 2L |
| beeverorum | NZ | |
| corrugatus | CAm | |
| dulciolens (Cu. melliolens) | NZ | |
| peraurantiacus | NZ | |
| pisciodorus | NZ |
Basidiomata medium-sized to large, cuphocyboid or sequestrate. Pileus 25–100 mm diam, viscid to glutinous, pale greyish brown to ochraceous, dark orange, or copper red, coarsely fibrillose to granulose, margin sometimes striate or sulcate. Lamellae/gleba pale cinnamon to argillaceous, sometimes with a violet tinge, crowded. Stipe cylindrical to clavate or rudimentary, often with a small rounded bulb, fibrillose, white, later darkening with red-brown tufts and squamules. Veil pale ochraceous, darkening to red-brown, fairly copious. Context white, slightly canescent or flavescent, sometimes marbled violet. Odour often aromatic or melleous. Alkaline reaction insignificant. Spores ellipsoid to subglobose, 9.5–17 × 7–11 μm, coarsely verrucose. In Fagaceae, Nothofagaceae, and Myrtaceae forests, South Pacific and Central America.
Notes — The bihemispherical section consists of taxa of vary-ing morphology with the type originally described in the genus Cuphocybe and four members sequestrate (formerly in genera Thaxterogaster and Protoglossum).
Cortinarius sect.Cuphocybe (R. Heim) Soop, comb. & stat. nov. (100 %)
Basionym. Cuphocybe R. Heim, Rev. Mycol. 16: 8. 1951.
Typus. Cuphocybe olivacea R. Heim.
MycoBank MB822941.
| elaiochrous (Cu. olivacea) | NZ | R |
| phaeomyxa | NZ | |
| C. sp. | Aus | 2L |
Notes — The former genus Cuphocybe is characterised by phlegmacioid taxa with a lacking cortina, which causes the veil to distribute as scales, tufts, and girdles along the full length of the stipe. Basidiomata are medium-sized to large, glutinous, coloration olive-brown to chocolate brown. Spores ellipsoid, large, 11–16 × 7–9 μm, moderately verrucose. In Nothofagaceae forests, South Pacific.
Cortinarius sect.Rapacea (E. Horak) Soop, comb. & stat. nov. (97 %)
Basionym. Rapacea E. Horak, Kew Bulletin 54: 789. 1999.
Paronym. C. series Rapacea Gasparini.
Typus. R. mariae E. Horak.
MycoBank MB822987.
| castoreus | NZ | R |
| mariae | NZ, Aus, N Guinea | R |
Notes — Basidiomata are medium-sized to large, robust, dry, sericeocyboid, with large spores (–18 μm). The section type, originally published in the genus Rapacea (named after C. rapaceus of similar colours), is unique in Cortinarius from its almost smooth spores, leaving an olive-yellow deposit. The boreal C. pinophilus appears as a sister with high support, but is morphologically not similar.
Section Crassi Melot (100 %)
Typus. C. crassus Fr., neotypus GB (ITS).
| crassus | Eur, NAm | R |
| eutactus | NZ |
Notes — Taxa in this small bihemispherical section evoke Phlegmacium, but were traditionally difficult to place in the taxonomy of the genus. Basidiomata are robust with a clavate stipe, dry, coloured pale to dark brown, with narrow, weakly verrucose spores and prominent cheilocystidia (cf. sect. Rubicunduli).
Cortinarius sect.Majestatici Soop, sect. nov. (89 %)
Typus. Descolea majestatica E. Horak, GB (ITS, LSU).
MycoBank MB822942.
| infrequens | SAm | |
| majestaticus | NZ | R |
Basidiomata rozitoid or sequestrate, medium-sized, viscid, cutis structure partly cellular. Pileus 30–60 mm diam, glutinous, dark yellow-brown, young with an olivaceous tinge, later darkening to umber, glabrous, margin striate to sulcate. Lamellae/gleba cinnamon, medium crowded. Stipe cylindrical or rudimentary, yellow-brown to pale yellowish grey, with thick yellow-brown fibrils and girdles, collar grey-brown, membranous. Veil yellow-brown, sparse to fairly copious. Context dirty white to yellowish white with an olivaceous tinge. Alkaline reaction red on cutis. Hypodermal cells in pileipellis with vesiculose ends. Spores amygdaloid, 12–14 × 7–8.5 μm, coarsely verrucose. In Nothofagaceae forests, South Pacific.
Notes — The type of this small austral section was originally described in the genus Descolea, but shares major characters with sect. Subcastanelli (Anderson & Orlovich 2016). The South American taxon is sequestrate.
Cortinarius sect.Rozites (P. Karst.) Soop, B. Oertel & Dima, comb. & stat. nov. (100 %)
Basionym. Rozites P. Karst., Bidrag Kannedom Finlands Natur Folk 32: 290. 1879.
Typus. C. caperatus Fr.
MycoBank MB822988.
| caperatus | Eur, NAm | R |
| emodensis | C Asia | 2L |
| fuegianus | SAm | G2016 |
| meleagris | NZ | |
| metallicus | Aus | 2L |
| similis | N Guinea | |
| (colombianus | CAm) |
Notes — Taxa published in the former genus Rozites are distributed over several clades, of which the above forms the bihemispherical type section. Basidiomata are characterised by a membranous inner veil, homologous with the cortina, amyloidal tissues, and strongly dextrinoid spores. One may also note that Rozites sect. Rozites (autonym) was described by Moser & Horak (1975).
Clade /Achroi (100 % in 2-loci tree)
| achrous | NZ | |
| cf. achrous | NZ | 2L |
| (elacatipus | NZ) | |
| (rugosiceps | NZ) |
Notes — Basidiomata are medium-sized to large, rozitoid, glutinous, hygrophanous, with a pale coloration and large spores. In Nothofagaceae forests.
Cortinarius sect.Subcastanelli Soop, sect. nov. (77 %)
Typus. Rozites castanellus E. Horak & G.M. Taylor.
MycoBank MB822943.
| canovestitus | NZ | |
| epiphaeus | NZ | |
| ohauensis | NZ | |
| subcastanellus (R. castanellus) | NZ | |
| taylorianus | NZ | |
| trichocarpus | NZ | |
| wallacei | NZ | R |
Basidiomata medium-sized to large of various habits: rozitoid, cuphocyboid, myxacioid, or sequestrate. Pileus 25–90 mm diam, dry to glutinous, red-brown to dark yellow-brown or tan, rarely with a violet tinge, coarsely fibrillose, sometimes with thick, greyish white to reddish tufts, margin striate to rimose when old. Lamellae/gleba cinnamon to yellow-brown or brownish pink, rarely violaceous, crowded to rather distant. Stipe cylindrical to clavate, often with a small rounded bulb, violaceous to white, pale grey, or pale yellow, with thick grey to brownish pink girdles, often with a striate, membranous collar. Veil greyish white to greyish yellow or reddish brown, rather sparse to copious. Context grey to pale grey-brown, ± marbled darker grey, yellow, or violet. Alkaline reaction reddish on cutis or insignificant. Spores ellipsoid to subamygdaloid, 8–14 × 5–8.5 μm, moderately to weakly verrucose. In Nothofagaceae forests, New Zealand.
Notes — This section is so far known only from New Zealand. Some of our C. epiphaeus samples were labelled C. napivelatus, a morphologically almost identical sequestrate fungus. Since the holotype of neither has been sequenced, it is too early to judge on their conspecificity, and we here use the dominant (and prioritary) epithet.
Cortinarius trichocarpus could be considered an agaricoid form of a morphospecies that includes the sequestrate C. ohauensis. Moreover, C. ohauensis is a late synonym of Austrogaster novaezelandiae, not yet combined into Cortinarius.
Cortinarius sect.Subhymenogaster Soop, B. Oertel & Dima, sect. nov. (100 %)
Typus. Hymenogaster sublilacinus A.H. Sm., GB (ITS).
MycoBank MB822944.
| brunnescens | NAm | |
| lilacinoides | NAm | 2L |
| subcaeruleus | NAm | |
| (Hymenogaster subolivaceus | NAm) |
Basidiomata sequestrate, globose to ovate or lobed. Peridium 10–55 mm diam, thin, dry, silky, whitish to lilac, flushing brown, purple, or bluish on manipulation. Gleba loculate, cinnamon to rusty brown. Columella white, tenous, dendrous to percurrent, or absent. Odour aromatic. Spores ellipsoid, 9–13 × 6.5–8 μm, weakly to moderately verrucose. In coniferous forests, North America.
Notes — The section is sister to sect. Caerulescentes. It has also been informally described as the genus Cortinogaster ined. See Smith (1966).
Cortinarius lilacinoides Soop, B. Oertel & Dima, nom. nov.
Basionym. Hymenogaster sublilacinus A.H. Sm., Mycologia 58(1): 108. 1966.
MycoBank MB823023.
Notes — The name C. sublilacinus is in use (Murrill 1944).
Cortinarius brunnescens (A.H. Sm.) Soop, B. Oertel & Dima, comb. nov.
Basionym: Hymenogaster brunnescens A.H. Sm., Mycologia 58(1): 111. 1966.
MycoBank MB822991.
Cortinarius subcaeruleus (A.H. Sm.) Soop, B. Oertel & Dima, comb. nov.
Basionym. Hymenogaster subcaeruleus A.H. Sm., Mycologia 58(1): 106. 1966.
MycoBank MB822992.
Section Cyanites Nespiak (100 %)
Typus. C. cyanites Fr., neotypus GB (ITS).
| boreicyanites | Eur | G2016 |
| cyanites | Eur | |
| spurcus | Eur | G2016 |
| violaceorubens | Eur | R |
Notes — A boreal section of similar species, characterised by dry and robust basidiomata, mainly of a greyish blue coloration and reddening context. The type has variously been placed in subg. Phlegmacium, Sericeocybe, and Telamonia.
Cortinarius sect.Austrocyanites Soop, sect. nov. (97 %)
Typus. C. austrocyanites Soop, GB (ITS, LSU).
MycoBank MB822945.
| austrocyanites | NZ, Aus |
| myxoclaricolor | SAm |
| poliotrichus ined. | NZ |
Basidiomata medium-sized to large, stipitocarpic, phlegmacioid or myxacioid. Pileus 25–100 mm diam, dry to viscid, grey-brown to yellow-brown or olive grey, coarsely grey fibrillose to squamulose. Lamellae greyish blue to argillaceous, crowded. Stipe dry to viscid, clavate with a rounded or marginate bulb, white to bluish. Veil pale grey to pale yellow or white with a ± violet tinge, rather sparse to copious. Context white, often marbled greyish blue to turquoise. Alkaline reaction red-brown to black on cutis, reddish lilac on lamellae. Spores ellipsoid to amygdaloid, 8–12 × 5–7 μm, moderately to rather weakly verrucose. In Nothofagaceae forests, South Pacific.
Cortinarius sect.Austrovaginati Soop, sect. nov. (100 %)
Typus. C. austrovaginatus Gasparini, GB (ITS, LSU).
MycoBank MB822946.
| austrovaginatus | Aus, NZ | |
| conei | NZ | |
| medioscaurus | NZ | R |
Basidiomata medium-sized, pileocarpic or sequestrate. Pileus 25–60 mm diam, viscid, yellow-brown to vinaceous brown or violaceous, disk ± red-brown, fibrillose, margin with white to yellow-brown tufts. Lamellae greyish violet to vinaceous, fairly crowded, sometimes sinuate or poorly developed. Stipe with a marginate bulb, sometimes volvate, silvery violet to lilac, coated white to yellowish on bulb. Veil white to greyish yellow, fairly copious. Context white to pale violet, taste sometimes bitter. Alkaline reaction reddish or insignificant. Spores ellipsoid to amygdaloid or citriform, 9–13 × 5.5–8 μm, coarsely verrucose. In Myrtaceae and Nothofagaceae forests, New Zealand, Australia.
Notes — The type species shows a tendency for sequestrate development, while C. conei is strictly sequestrate.
Cortinarius sect.Verniciori Soop, sect. nov. (99 %)
Typus. C. verniciorum Soop, GB (ITS).
MycoBank MB822947.
| dulcamarus | NZ | |
| verniciorum | NZ | |
| C. sp. | NZ | 2L |
Basidiomata medium-sized to small, stipitocarpic, phlegmacioid or telamonioid. Pileus 15–50 mm diam, viscid, apricot to yellow-brown with a ± orange disk, glabrous to finely fibrillose. Lamellae white to pale grey-brown, medium crowded. Stipe cylindrical to ± clavate, whitish to pale grey-brown, flavescent. Veil white, sparse. Context pale yellow, marbled yellow-brown. Odour melleous or insignificant, taste acerbic to bitter. Alkaline reaction red to red-brown. Spores fusoid to subamygdaloid, 5.5–8 × 3–4 μm, weakly verrucose. In Nothofagaceae forests, New Zealand.
Section Infracti (Kühner & Romagn.) Moënne-Locc. & Reumaux (100 %)
Typus. C. infractus (Fr.: Fr.) Fr., neotypus GB (ITS).
| anfractoides | Eur | JEC |
| ayanamii | Eur | JEC |
| infractiflavus | Eur, NAm | 2L |
| infractiflavus II | Eur, India | JEC |
| infractus | Eur, NAm | |
| infractus II | Eur | JEC |
| infractus III | Eur, NAm | 2L |
| maculatocaespitosus | Eur | R |
| obscurocyaneus | Eur | |
| persoonianus | Eur | |
| cf. infractus | NAm | 2L |
Notes — A boreal section of phlegmacioid, stipitocarpic taxa, whose basidiomata present dark colours with a more or less pronounced olive component, growing with a wide range of coniferous and frondose hosts. It is further characterised by the presence of the alkaloid infractopicrin (Stensrud et al. 2014). The New Zealand species C. pholiotellus is closely related, but is not included due to morphological differences.
Section Subtorti Brandrud & Melot (100 % in 2-loci tree)
Typus. C. subtortus Fr., neotypus GB (ITS).
| subtortus | Eur, NAm | R |
| subtortus II | NAm | 2L |
| (amurceus | Eur) |
Notes — A small boreal, phlegmacioid or myxacioid section, characterised by olivaceous-yellow tints and the presence of the alkaloid quinoline (Stensrud et al. 2014). It often appears as a sister to sect. Delibuti.
Cortinarius sect.Purpurelli Soop, sect. nov. (100 %)
Typus. Rozites violacea E. Horak, Beih. Nova Hedwigia 52: 516. 1975.
MycoBank MB822950.
| ochraceoazureus | SAm | |
| purpurellus (R. violacea) | SAm | |
| sarmienti (togularis) | SAm | 2L |
| C. sp. | SAm | 2L |
Basidiomata stipitocarpic, rozitoid, or sequestrate. Pileus 30–80 mm diam, viscid, violet to greyish purple or yellow-brown, margin sometimes with violaceous tufts. Lamellae white to pale brown or rusty yellow, rather distant, sometimes sinuous/wrinkled and bifurcate. Stipe cylindrical to clavate, violet to whitish, brunnescent, silky, girdled or collared. Veil white to violet, brunnescent, fairly copious. Context white to pale yellowish, brunnescent. Odour rarely strong, sweetish. Alkaline reaction insignificant. Spores ovoid to amygdaloid, 8–11 × 5.5–8 μm, weakly verrucose. In Nothofagaceae forests, South America.
Notes — Several members were described in the former genera Thaxterogaster and Rozites (Horak & Moser 1965).
Cortinarius sect.Entheosi Soop, sect. nov. (87 % in PhyML tree)
Typus. C. entheosus Soop, GB (ITS, LSU).
MycoBank MB824054.
| aerugineoconicus | NZ |
| entheosus | NZ |
Basidiomata medium-sized, stipitocarpic, phlegmacioid or myxacioid, with a vivid coloration. Pileus 25–60 mm, viscid, tan to yellowish with a brownish orange disk, or greenish blue, glabrous to finely fibrillose. Lamellae blue to violet, crowded. Stipe cylindrical to ± fusoid, dry or viscid, white to pale blue, fibrillose. Veil white to pale violet, fairly copious. Context grey buff to yellowish, marbled violet. Alkaline reaction blue or insignificant. Spores ellipsoid to amygdaloid, 7.5–10 × 4.5–6 μm, moderately to coarsely verrucose. In Nothofagaceae forests, New Zealand.
Cortinarius sect.Salmaster Soop, sect. nov. (81 %)
Typus. C. salmaster Gasparini.
MycoBank MB822951.
| salmaster | Aus |
| salmastrium | NZ |
Basidiomata medium-sized to small, stipitocarpic, phlegmacioid or telamonioid, greenish. Pileus 20–35 mm diam, viscid, dark olive brown to greyish green, glabrous to finely fibrillose. Lamellae grey to pale grey-brown, medium crowded. Stipe cylindrical, often rooted, silky white or pale green-grey to pale green-blue. Veil yellowish white to brownish, sparse. Context white to green-grey or lilac. Alkaline reaction insignificant. Spores ellipsoid, 7–8 × 4–5.2 μm, moderately verrucose. In Myrtaceae forests, South Pacific.
Section Malvacei M.M. Moser (87 %)
Typus. C. malvaceus E. Horak.
| malvaceus | SAm | R |
| cf. castaneiceps | NZ | |
| (austroalbidus | SAm) |
Notes — Basidiomata are medium-sized to small, stipitocarpic, phlegmacioid or myxacioid, with a pale lilac coloration. In Nothofagaceae forests.
Clade /Ardesiaci (100 %)
| ardesiacus | Aus |
| cf. ardesiacus | NZ |
Notes — Basidiomata are medium-sized, stipitocarpic, phleg-macioid, coloration grey-brown to brown. In Myrtaceae forests.
Clade /Cartilaginei (100 %)
| cartilagineus | NZ |
| C. sp. | NZ |
Notes — Basidiomata are sequestrate or cuphocyboid, coloration ochraceous. In Nothofagaceae forests.
Clade /Australienses (100 %)
| australiensis | Aus | R |
| cf. australiensis | NZ | R |
Notes — Basidiomata are large, whitish, viscid, with a peronate veil. Spores 9–12 × 5.5–6.5 μm, amygdaloid. The taxa have also been attributed to the genus Rozites. In Myrtaceae forests.
5. Anomaloid sections
Three sections are bihemispherical and the basidiomata are characterised by a yellowish or reddish veil, a relatively slender habit, and rounded spores. The sections are represented by two lineages in Fig. S2 of Garnica et al. (2016).
Section Anomali Konrad & Maubl. (100 %)
Typus. C. anomalus (Fr.: Fr.) Fr., neotypus GB (ITS).
| albocyaneus | Eur | G2016 |
| anomalellus | Eur | 2L |
| anomalovelatus | NAm | |
| anomalus (azureus) | Eur, NAm | R |
| azureovelatus | Eur | Dima et al. (in prep.) |
| barlowensis | NAm | |
| caesiifolius | NAm | 2L |
| caninus | Eur, NAm | |
| durifoliorum | NZ | R |
| lebretonii | Eur | Dima et al. (in prep.) |
| lepidopus | Eur | 2L |
| pastoralis (anomalus subsp. campestris) | Eur | 2L |
| rattinoides | NZ | |
| sclerophyllorum | Aus | |
| sericeolazulinus | NAm | |
| suecicolor | NZ | R |
| tabularis | Eur | 2L |
| tristis | SAm | G2016 |
| xanthocephalus | Eur | G2016 |
| cf. anomalus | NAm | 2L |
| cf. azureus | NAm | 2L |
| cf. caninus | NAm | 2L |
| cf. durifoliorum | NZ | 2L |
| cf. suecicolor | NZ |
Notes — This section has sometimes been presumed close to the morphologically similar sect. Delibuti (below), a relation that is not borne out by our analysis. The New Zealand species C. eunomalus appears as basal to this and the following two sections. See further Dima et al. (2016).
Section Bolares Kühner & Romagn. (100 %)
Typus. C. bolaris (Fr.: Fr.) Fr., neotypus GB (ITS).
| bolaris | Eur, NAm, CAm | R |
| cf. sclerophyllorum | NZ |
Notes — The section is bihemispherical and consists of anomaloid fungi with a brightly red veil.
Section Spilomei (Bidaud, Moënne-Locc. & Reumaux) Cons., D. Antonini & M. Antonini (96 %)
Typus. C. spilomeus (Fr.: Fr.) Fr., neotypus GB (ITS).
| ferrusinus | Eur | Ballarà et al. (2017) |
| spilomeus | Eur | |
| cf. azureus | NAm | |
| (depauperatus | Eur) |
Notes — The section is boreal and consists of anomaloid fungi with a reddish veil.
Section Delibuti (Fr.) Sacc. (96 %)
Typus. C. delibutus Fr.
| calaisopus | NZ, Aus | |
| calaisopus II | NZ | 2L |
| delibutus | Eur, NAm | 2L |
| delibutus II | Eur, NAm | R |
| illibatus | Eur | 2L |
| illitus | SAm | G2016 |
| rotundisporus | Aus, NZ | |
| salor | Eur, NAm | R |
| tessiae | NZ | |
| cf. rotundisporus | NZ | G2016 |
| cf. salor | NAm | 2L |
| (betulinus | Eur) | |
| (largodelibutus | Eur) | |
| (transiens | Eur) |
Notes — The species are recognised by the same characters as in sect. Anomali, exhibiting in addition a viscid to glutinous veil. Basidiomata are coloured yellow, blue, or green, where the green pigment appears to occur mainly in the South Pacific. Unexpectedly, the European taxon C. emunctus is a singleton outside the section.
6. Cortinarius sect. Gigasperma (E. Horak) Soop & B. Oertel, comb. & stat. nov.
Basionym. Gigasperma E. Horak, New Zealand J. Bot. 9: 491. 1970.
Typus. G. crypticum E. Horak, GB (ITS).
MycoBank MB822990.
| crypticus | NZ |
Cortinarius crypticus (E. Horak) Soop & B. Oertel, comb. nov.
Basionym. Gigasperma crypticum E. Horak, New Zealand J. Bot. 9: 491. 1970.
MycoBank MB822995.
Notes — A small sequestrate fungus with very large, thick-walled, smooth spores. Two other species have been described in Gigasperma, none of which, however, is part of Cortinarius (Index Fungorum).
7. Myxacioid sections
The subsequent four sections, along with Cortinarius bellus (New Zealand), form a robust clade, which contains the type section of subg. Myxacium. They are represented by the main Myxacium lineage in Fig. S2 of Garnica et al. (2016). Also the sequestrate C. porphyroideus from New Zealand may possibly be included.
Section Myxacium (Fr.) Gillot & Lucand (100 %)
Typus. C. collinitus (Pers.: Fr.) Fr.
| alpinus | Eur, NAm | G2016 |
| collinitus | Eur, NAm | R |
| collinitus II | Eur, NAm | 2L |
| fennoscandicus | Eur | 2L |
| mucosus | Eur, NAm | |
| pingue | NAm | |
| septentrionalis | Eur, NAm | 2L |
| stillatitius II | Eur | 2L |
| trivialis | Eur, NAm | |
| vernicosus | NAm | 2L |
| (grallipes | Eur) |
Notes — A boreal section of glutinous taxa, presenting white, brown, and violet colours. Cortinarius pingue is sequestrate.
Section Defibulati M.M. Moser (75 %)
Typus. C. elatior Fr.
| basipurpureus | Aus | |
| brunneoalbus | NAm | Ariyawansa et al. (2015) |
| costaricensis | CAm | 2L |
| cuphocyboides | NZ | |
| gymnocephalus | NZ | |
| mucifluus | Eur | 2L |
| pavelekii | NAm | 2L |
| phlegmophorus | India | G2016 |
| seidliae | NAm | |
| stillatitius | Eur, NAm | R |
| subviolaceus (P. violaceum) | Aus | G2016 |
| vanduzerensis | NAm | |
| (elatior | Eur) | |
| (pumilus | Eur) |
Notes — A widely distributed section of taxa, morphologically similar to sect. Myxacium and Cuphocybe. Two species are sequestrate. All taxa are characterised by hyphae without clamp connections.
Cortinarius sect.Marmorati Soop, sect. nov. (62 %)
Typus. C. marmoratus E. Horak, GB (ITS).
MycoBank MB822952.
| marmoratus (anauensis) | NZ | |
| purpureocapitatus ined. | NZ | 2L |
| vitreofulvus | NZ | R |
| vitreopileatus | NZ | |
| cf. vitreofulvus | NZ | |
| cf. vitreofulvus II | NZ | |
| (viscostriatus | NZ) |
Basidiomata medium-sized, myxacioid or sequestrate. Pileus, 25–60 mm diam, glutinous, white to yellow-brown, purple-brown, chocolate-brown, or lilac, disk often paler grey to ochra-ceous, glabrous. Lamellae white, greyish or deeply violet, medium crowded. Stipe cylindrical to slightly clavate, sometimes with a small rounded bulb, viscid, white to violaceous-grey. Veil pale violaceous, ± brunnescent, sparse. Context grey-white, sometimes marbled violet. Alkaline reaction insignificant or ± red-brown on cutis. Spores amygdaloid, 11–15 × 6.5–8 μm, moderately to strongly verrucose. Clamp connections present. In Nothofagaceae forests, New Zealand.
Notes — The section is so far endemic to New Zealand, but has some morphological similarity with sect. Myxacium.
Cortinarius sect.Cuphomorphi Soop, sect. nov. (97 %)
Typus. C. cuphomorphus Soop, GB (ITS, LSU).
MycoBank MB822953.
| cuphomorphus | NZ | |
| juglandaceus | NZ | |
| violaceocystidiatus ined. | NZ | 2L |
Basidiomata medium-sized to small, cuphocyboid, myxacioid, or sequestrate. Pileus 15–55 mm diam, viscid, pale grey-brown to dark brown or violet, ± provided with fibrils and squamules when young, glabrous when older. Lamellae grey-blue, medium crowded to rather distant. Stipe cylindrical, sometimes with a small rounded bulb, dry to viscid, white to grey-brown, young with a violet tinge, sometimes with grey or yellowish squamules over the whole length. Veil pale yellow to ochraceous, fairly copious. Context white to pale violet, marbled grey-blue, odour sweetish, sometimes strong. Alkaline reaction insignificant. Spores amygdaloid, 10–12.5 × 6–8 μm, rather strongly verrucose. Clamp connections sparse or absent. In Nothofagaceae forests, New Zealand.
Section Cycnei Soop (87 %)
Typus. C. cycneus E. Horak.
capitellinus SAm Salgado Salomón et al. (2018)
| cucumeris | NZ | |
| cycneus | NZ | |
| lubricanescens | NZ | R |
| magellanicus | SAm | |
| roblerauli | SAm | 2L |
| (cf. magellanicus | N Caledonia) |
Notes — A section of myxacioid taxa, confined to the Southern Hemisphere (Soop 2016). Cortinarius magellanicus has been reported several times from New Zealand, but our analyses show that it is a violet form of C. lubricanescens. See further Salgado Salomón et al. (2018).
Section Vibratiles Melot (91 %)
Typus. C. vibratilis (Fr.: Fr.) Fr.
| alboamarescens | Eur | 2L |
| barbatus | Eur | |
| causticus | Eur, NAm | 2L |
| causticus II | NAm | 2L |
| croceocoeruleus | Eur | |
| electridius ined. | NZ | 2L |
| emollitoides | Eur | 2L |
| melleomitis | SAm, NZ | R |
| microspermus | Eur | |
| ochroamarus | Eur | 2L |
| pluviorum | Eur | |
| pluvius | Eur | |
| psilomorphus | NZ | |
| vibratilis | Eur, NAm | |
| (decumbens | Eur) | |
| (emollitus | Eur) | |
| (galeobdolon | Eur) | |
| (gemmeus | NZ) | |
| (ochroleucus | Eur) |
Notes — This section is bihemispherical, consisting of glutinous fungi of a relatively modest size. Many of the taxa are further distinguished by a bitter context. We have only examined C. melleomitis samples from New Zealand, whereas the type is described from Patagonia.
Section Archeriani M.M. Moser & E. Horak (88 % in PhyML tree)
Typus. C. archeri Berk.
| archeri | Aus | |
| holojanthinus | SAm | 2L |
| cf. taylorianus | NZ | 2L |
Notes — A small South Pacific section of glutinous agaricoid or sequestrate fungi with violaceus and whitish colours.
Clade /Lustrabiles (100 %)
| badiohepaticus ined. | NZ | |
| lustrabilis | Eur | R |
Notes — A small bihemispherical clade of rare taxa that resemble those in sect. Vibratiles. Basidiomata are medium-sized to small, myxacioid, coloration red-brown to yellow-brown. In Fagaceae and Nothofagaceae forests.
8. Telamonioid sections
8.1. Subgenus Telamonia s.str.
This large monophyletic entity was recovered as the main Telamonia lineage in Fig. S2 of Garnica et al. (2016). It contains the vast majority of species described in the subgenus, and appears to be endemic for the Northern Hemisphere. Basidiomata are dry and generally present a stipitocarpic habit with a hygrophanous context. Colours are usually drab brownish or greyish, occasionally with a violet component. Many of the species are of a modest size. Recently many new species were described from the northern coniferous taiga belt, making this the most diversified region for Telamonia known to date.
The phylogeny reveals a number of clades, many of which are recovered as traditional sections. These are not further discussed in this study, having been extensively documented in several dedicated efforts (e.g., Kytövuori et al. 2005, Niskanen 2008, Niskanen et al. 2009, 2011, 2012, 2013), some of which are currently being pursued.
8.2. Other telamonioid sections
This is a polyphyletic assembly of sections, some bihemispherical. A number of the northern taxa have traditionally been included in subg. Telamonia (s.lat.), and their basidiomata look like what is known as ‘typical Telamonia’. One of these lineages (Obtusi) was recovered in Fig. S2 of Garnica et al. (2016). Southern taxa, on the other hand, often exhibit several deviating characters, such as bright colours and a positive alkaline reaction.
Section Renidentes Rob. Henry ex Moënne-Locc. & Reumaux (92 %)
Typus. C. renidens Fr.
| parahumilis | SAm | |
| renidens | Eur, NAm | R |
Notes — The section is bihemispherical. Cortinarius renidens appears to be unique in the genus by lacking both universal veil and cortina. Garnica et al. (2005) and Stensrud et al. (2014), report a strong support for including sect. Austroduracini (below), but we prefer to keep them as separate entities since their affinity is inconsistent in our study.
Cortinarius sect.Austroduracini Soop & Dima, sect. nov. (87–90 % in 2-loci tree)
Typus. C. austroduracinus M.M. Moser.
MycoBank MB822958.
| austroduracinus | SAm | R |
| pyrrhomarmarus ined. | NZ | 2L |
| viridibasalis | SAm | |
| viscincisus | NZ |
Basidiomata small, telamonioid, yellow-brown to red-brown. Pileus 10–40 mm diam, viscid, yellow-brown to dark red-brown, finely fibrillose. Lamellae greyish or pale cinnamon to dark yellow-brown, medium crowded. Stipe cylindrical, pale brown to greyish yellow with white to dark yellow-brown thin bands or girdles. Veil white to dark yellow-brown, sparse to copious. Context pale brown to yellowish, often marbled brownish. Alkaline reaction insignificant. Spores ellipsoid, 7–9.5 × 4.5–6, moderately to fairly coarsely verrucose. In Nothofagaceae forests, South America, New Zealand.
Cortinarius sect. Camphorati (Liimat., Niskanen & Ammirati) Soop, B. Oertel & Dima, stat. nov. (100 %)
Basionym. Cortinarius subg. Camphorati Liimat., Niskanen & Ammirati, Index Fungorum 256: 2. 2015. IF551459.
Synonym. Cortinarius sect. Camphorati Liimat. & Niskanen, in Niskanen (2008: 19), nom. inval.
Typus. C. camphoratus Fr.
MycoBank MB824052.
| camphoratus | Eur, NAm | |
| dysodes | NZ | |
| putorius | NAm | 2L |
| tasmacamphoratus | Aus | |
| cf. dysodes | NZ | 2L |
| (austrotorvus | Aus) |
Notes — This taxon is bihemispherical. Basidiomata are dry with grey-brown and violet colours, but are mainly characterised by a strong, obnoxious odour reminding of acetylene, which appears to be synapomorphic. See further Niskanen (2008) and Niskanen et al. (2015).
Section Obtusi Melot (81 %)
Typus. C. obtusus (Fr.: Fr.) Fr.
| acutovelatus | Eur | 2L |
| acutus | Eur, NAm | |
| amblyonis | NZ | |
| basifibrillosus ined. | NZ | |
| ceraceus | Eur, NAm | 2L |
| conopileus | India | G2016 |
| cystidiocatenatus | Aus | |
| leucocephalus | NZ | G2016 |
| obtusus | Eur, NAm | |
| pachynemeus | SAm | |
| pseudocandelaris | Eur | 2L |
| pseudoduracinus | Eur | 2L |
| rigens | Eur | 2L |
| squamiger | SAm | 2L |
| tenellus | SAm | 2L |
| walpolensis | Aus | G2016 |
| cf. amblyonis | NZ | R |
| cf. levisporus | Aus | G2016 |
| C. sp. I | NZ | 2L |
| C. sp. II | NZ | 2L |
| C. sp. III | NZ | 2L |
| C. sp. IV | NZ | 2L |
| C. sp. V | NZ | R |
| (albovariegatus | Eur) | |
| (fibrillosus | Aus) | |
| (trossingenensis | Eur) |
Notes — A bihemispherical section of small brownish fungi, characterised by the frequent presence of vesiculose cheilocystidia. Two of the austral species are sequestrate.
Section Laeti Melot (97 %)
Typus. C. laetus M.M. Moser.
| badiovinaceus | Eur, NAm | |
| bulliardioides | Eur | G2016 |
| floccopus | Eur | G2016 |
| laetiluteinus ined. | NZ | |
| laetus (detonsus?) | Eur, NAm | |
| lanceolatus | NZ | 2L |
| ochrophyllus | Eur | |
| subfloccopus | Eur, NAm | 2L |
| veregregius | Eur | G2016 |
| waiporianus | NZ | |
| C. sp. I | NZ | 2L |
| C. sp. III | NZ | 2L |
| C. sp. IV | NZ | 2L |
| C. sp. V | NZ | R |
| cf. fasciatus | Eur | R |
| cf. pauperculus | Eur | 2L |
| (bayeri | Eur) | |
| (fulvescens | Eur, NAm) | |
| (fulvescentoides | Eur, NAm) | |
| (nymphatus | Eur, NAm) | |
| (pseudobulliardioides | Eur, NAm) | |
| (tenuifulvescens | Eur, NAm) |
Notes — A bihemispherical section of medium-sized to small fungi that often present a yellowish or reddish veil. It appears as a sister to sect. Obtusi. See also Garrido-Benavent et al. (2014), Niskanen (2008), Hyde et al. (2016).
Cortinarius sect. Illumini (Liimat., Niskanen & Kytöv.) Soop, B. Oertel & Dima, stat. nov. (80 %)
Basionym. Cortinarius subg. Illumini Liimat., Niskanen & Kytöv., Index Fungorum 256: 2. 2015. IF551474.
Synonym. Cortinarius sect. Illumini Liimat., Niskanen & Kytöv. in Niskanen (2008: 19), nom. inval.
Typus. C. illuminus Fr., neotypus GB (ITS, LSU).
MycoBank MB824053.
| badiovinaceus II | Eur | |
| balaustinus | Eur | G2016 |
| cypripedii | NZ | R |
| illuminus | Eur, NAm | |
| microglobisporus | Eur |
Notes — A bihemispherical taxon of medium-sized fungi displaying a vividly red-brown pileus and rounded spores. See further Niskanen (2008) and Niskanen et al. (2015).
Cortinarius sect.Carbonelli Soop, sect. nov. (80 %)
Typus. C. carbonellus Soop, GB (ITS, LSU).
MycoBank MB822959.
| carbonellus | NZ | R |
| rattinus | NZ |
Basidiomata small, telamonioid, grey-brown to dark brown. Pileus 10–45 mm diam, dry, dark grey-brown to bluish grey, or purple-brown, later umber, finely fibrillose. Lamellae purple-brown to violet, greyish blue, or dark grey soon ± black, fairly crowded. Stipe cylindrical to clavate, silvery grey to yellowish grey with a violet tinge. Veil violet to red-brown, sparse. Context greyish to vinaceous brown, marbled violet. Alkaline reaction orange to reddish lilac in context, cherry red on lamellae. Spores ellipsoid to ± amygdaloid, 7–10 × 4–5.5 μm, moderately to rather weakly verrucose. In Nothofagaceae forest, New Zealand.
Unsupported group Pseudoxenosmatae
This austral group is formed by sect. Carbonelli and the following taxa:
| areolatoimbricatus | Aus | R |
| cupreonatus | NZ | R |
| exlugubris | NZ | |
| punctatisporus | SAm | 2L |
| vernicifer | NZ |
Notes — The group mainly contains of dry, often robust, sericeocyboid fungi. It receives low support, except in the PhyML tree (89 %). It contains several members of sect. Xenosmatae (see Soop 2002). The latter is not recovered in our study, which reveals its type C. xenosma as a singleton.
Cortinarius sect.Paraxanthi Soop, sect. nov. (100 %)
Typus. C. paraxanthus Soop, GB (ITS, LSU).
MycoBank MB822960.
| citribasalis | NZ |
| paraxanthus | NZ |
Basidiomata small, telamonioid, yellow-brown to olivaceous. Pileus 15–45 mm diam, dry, olive-brown to orange-brown or mahogany brown, often with an umber disk, finely fibrillose, margin yellow, often with an olive shade. Lamellae yellow-brown to orange, rather distant. Stipe cylindrical, lemon-yellow to yellow-green, base with white or yellow rhizomorphs. Veil greenish yellow, blushing red-brown, sparse. Context brown-yellow or orange to grey-brown, sometimes with an olive tinge. Alkaline reaction reddish or insignificant. Spores ellipsoid to amygdaloid, 7–9.5 × 4–6 μm, moderately to weakly verrucose. In Nothofagaceae forest, New Zealand.
Cortinarius sect.Luteini Soop, sect. nov. (100 %)
Typus. C. luteinus Soop. GB (ITS, LSU).
MycoBank MB822961.
| luteinus | NZ |
| palissandrinus | NZ |
Basidiomata small, telamonioid, yellow to red-brown. Pileus 10–30 mm diam, dry, dark yellow to brownish orange or mahogany red, later with a yellow-orange disk, finely fibrillose. Lamellae dark yellow to orange, brick red, or orange-red, distant. Stipe cylindrical, yellow to greyish yellow or pale brownish orange, sometimes ± citrinous, with thin, yellowish fibrils. Veil yellow, later darkening with a reddish tone, sparse. Context greyish yellow, marbled darker yellow. Odour raphanoid. Alkaline reaction weak or red on cutis and lamellae. Spores obtusely ellipsoid, 6.5–8.5 × 4.5–6 μm, moderately verrucose, marginal elements vesiculose, 20–35 × 9–12 μm. In Nothofagaceae forest, New Zealand.
Clade /Minilaci (99 %)
| minilacus | NZ | |
| C. sp. I | NZ | |
| C. sp. II | NZ | 2L |
Notes — This austral clade consists of telamonioid fungi with yellow-brown lamellae and subglobose spores. In Myrtaceae and Nothofagaceae forest.
Clade /Chrysoconii (96 %)
| chrysoconius | NZ |
| cf. chrysoconius | NZ |
Notes — Basidiomata are small, telamonioid, coloration yellow with a squamulose pileus. In Nothofagaceae forests.
DISCUSSION
Higher ranks
In the present study we are not formally introducing taxonomic structures of higher rank than section. Robust phylogenetic support for higher ranks, such as subgenera, would presumably require a dataset built on more loci than the current nrITS, nrLSU, rpb1, and rpb2 (cf. Frøslev et al. 2005). It may nevertheless be appropriate, as part of a discussion, to review a few major tentative lineages suggested by our phylograms, albeit with modest support in some cases. The history of a couple of lineages is reviewed in more detail.
– Anomali — This lineage would include sect. Anomali, Bolares, Camphorati, and Spilomei.
– Calochroi — This taxon would form an own lineage with clade /Arcifolii.
– Cortinarius — The type section of the genus would form its own lineage.
– Crassi — This lineage would include sect. Crassi and Rubicunduli.
– Delibuti — This lineage would include sect. Delibuti and Subtorti.
– Dermocybe — This lineage would include sect. Dermocybe, Cruentoides, and Pauperae.
– Icterinula — This lineage would include sect. Walkeri, Chrys-mata, Rubrobasales, Ignelli, and clade /Orixanthi, as well as the singleton species in the unsupported group Icterinula.
– Leprocybe — This lineage would include sect. Leprocybe, Persplendidi, and Veronicae. It is sister to the Dermocybe lineage.
– Limonii and Callistei — The two sections form their own lineages in our phylogeny. The first name was introduced by Kühner & Romagnesi (1953) for a group based on the European Cortinarius limonius. Moser (1969) promoted Limonii to a section, expanded it with a number of boreal taxa, and attributed it to his new subg. Leprocybe. But he wisely refrained to include the morphologically similar C. callisteus, which had originally been present. Orlovich & Oliver (2002) found that C. rubrocastaneus, a species from New Zealand, is also part of the section. Our analysis now shows that at least eight recently discovered species belong to the section, making New Zealand the most diversified area for sect. Limonii known today (Soop et al. 2018). Cortinarius callisteus, on the other hand, is part of the bihemispherical sect. Callistei, a separate lineage, consistent with the concept of subg. Callistei (Niskanen et al. 2016).
– Multiformes — This lineage would include sect. Cremeolinae, Malvacei, Multiformes, and Vibratiles.
– Myxacium — This lineage would include sect. Cuphomorphi, Defibulati, Marmorati, and Myxacium, as well as a number of sequestrate species from the South Pacific.
– Phlegmacium I — This lineage would include sect. Arguti, Claricolores, Elastici, Percomes, Phlegmacioides, Phlegmacium, clades /Caligati, /Obsoleti, /Rhizophori, /Varii, and a number of singleton species. Virtually all species in this lineage have a stipitocarpic development.
– Phlegmacium II — This lineage would include sect. Amoenolentes, Aureocistophili, Caerulescentes, Glaucopodes, Subhymenogaster, clades /Camptori, /Dionysae, /Eucaerulei, /Glaucocephali, /Subolivascentes, and a number of singleton species. Virtually all species in this lineage have a pileocarpic development.
– Rozites — This lineage would include sect. Cuphocybe, Majestatici, Rozites, Subcastanelli, clade /Achroi, and several rozitoid species from the South Pacific.
– Scauri — This lineage would include sect. Purpurascentes and Scauri, as well as one singleton species from the South Pacific.
– Telamonia — This lineage would include Telamonia s.str., and possibly sect. Purpurelli, which is consistently basal to the former but morphologically different. The subgenus goes back to Fries and circumscribes today, in its traditional sense, at least 900 taxa. These are morphologically fairly homogeneous, presenting a dry, more or less hygrophanous pileus and generally drab brown, grey, or violet colours. Høiland & Holst-Jensen (2000) and Peintner et al. (2004) showed that a large core portion of the subgenus was monophyletic, leaving the common telamonioid sect. Obtusi in a separate lineage. The core portion, here termed Telamonia s.str., seemed to be confined to the Northern Hemisphere, as later confirmed by Garnica et al. (2005, 2016). These and our own studies show that not only Obtusi but several other telamonioid taxa (Camphorati, Illumini, Laeti, Renidentes) are positioned outside Telamonia s.str. and are in fact bihemispherical (cf. Niskanen 2008). Originally, also a number of austral species were classified as Telamonia (Moser & Horak 1975, Grgurinovich 1997, Soop 1998, 2001, 2005, Gasparini & Soop 2008), in many cases despite traits not usually associated with what may be called a ‘typical Telamonia’. Our analysis shows that some of these species form endemic sections (e.g., Austroduracini) in the South Pacific, genetically well separated from the northern core clade, and no published southern species has so far been shown to belong to Telamonia s.str.
– Telamonioidae — This lineage would include part of the remains of Telamonia s.lat.: sect. Obtusi, Laeti, and Illu-mini (but neither Camphorati nor Renidentes), as well as the clade /Minilaci. It is sister to the Dermocybe-Icterinula-Leprocybe complex, an interesting topology that was also implicitly shown by the 5-loci phylogram of Garnica et al. (2016).
Traditional vs phylogenetic sections
In several cases traditional sections were shown by our study to be polyphyletic, while often retaining a monophyletic core of species around the type. In other cases the sections were broken up into disparate lineages. For example, the boreal sect. Claricolores was earlier defined by the fusoid spore shape and caespitose growth, and then contained notably Cortinarius claricolor, C. variegatus, and C. turmalis (cf. Brandrud et al. 1989, 1992, 1994, 1998, 2012). Our analysis recovered C. variegatus as a singleton, while C. turmalis unexpectedly forms a well-supported clade with the austral C. picoides. The new sect. Turmales in fact retains the two mentioned character states, while the emended sect. Claricolores (Brandrud et al. 2013) shows characters like a sulcate pileus margin, and includes species such as C. praestans.
The relatively recent discovery of many new species from the Southern Hemisphere has resolved a number of singletons into small clades, some of which are here described as new bihemispherical sections, like Turmales mentioned above. Another example of this pattern is the boreal Cortinarius crassus, traditionally considered an odd entity, difficult to place in the taxonomy, and long the only member of its sect. Crassi. But as in the case of Turmales, the discovery of the austral C. eutactus has revealed Crassi as bihemispherical. The two species share the habit and the presence of remarkable cheilocystidia. The pattern is replicated by the boreal C. rubicundulus having now found a southern ‘partner’ in C. subgemmeus, with which it shares the characters of cheilocystidia and a flavescent context.
In larger clades the core of species sometimes lies in one of the hemispheres. Cortinarius sect. Pauperae, for example, consists almost exclusively of austral species, C. olivaceofuscus in Europe (cf. Høiland 1983) being one of the notable exceptions. Conversely, sect. Phlegmacioides, with more than 20 boreal species, contains the single Australian species C. lavendulensis. Other sections, such as Purpurascentes and Anomali exhibit a fairly even distribution between the hemispheres. An extreme case is sect. Defibulati, which is found in Europe, North and Central America, the South Pacific, and India. Among the telamonioid sections Anomali and Obtusi constitute other widely distributed taxa.
A number of lineages appear to occur exclusively in either hemisphere (Soop & Gasparini 2011). In our analysis a conspicuous number of phlegmacioid clades are confined to the North. In the Introduction, we mentioned Calochroi s.lat., but also Multiformes, Claricolores, Riederi, Glaucopodes, Arguti, and Caerulescentes, just to name a few, belong to this category. In the South the endemic sect. Cremeolinae is closely related to the boreal Multiformes, to which there is also a strong morphological resemblance. Other section pairs exhibiting a similar biogeographical pattern are Leprocybe-Persplendidi and Myxacium-Marmorati.
Biogeographical section concept
Even though a number of sections are bihemispherical, our study corroborates the fact that no Cortinarius species is known to occur naturally in both hemispheres (pers. obs., and cf. Harrower et al. 2015a: 705). This could be explained by the strict ectomycorrhizal host specificity within the genus (Wang & Qiu 2006, and cf. also Brandrud 1996 and Kytövuori et al. 2005). Few, if any, of the putative host genera are naturally present in the temperate regions of both hemispheres (the exceptions may be Quercus and Alnus in South America). A strict analysis of this biogeographical relationship would involve the history of plant evolution, which is beyond the scope of the present study. Such a study was presented by Wilson et al. (2017) on the subject of the genus Laccaria. But whereas Wilson et al. (2017) report a distinct north-south split into two major clades for Laccaria, our topology shows a mosaic of smaller, intermixed, boreal and austral clades for Cortinarius, similar to the clade structure of Garnica et al. (2016).
Looking closer at this structure, one notes that a number of potential section clades can be neatly split into two subclades, one endemic for each hemisphere. This topology often exhibits two important patterns: (a) one can significantly boost the bootstrap support by excluding one of the subclades from the section; and (b) the austral subclade tends to be ‘basal’ in the sense that its branch is shorter than the boreal one. The interesting pattern (b) and its hypothetical connection with the evolution of the genus would be a subject for future research.
With reference to (a), one is faced with the choice of conceiving the clade as one bihemispherical section with lower support, or as two well-supported, geographically separated sections. Where the two subclades are morphologically similar we have sometimes opted for the first choice. For example, we chose to retain a bihemispherical sect. Illumini (with a lower support) due to phenotypical similarity across the clade. A contrary example is the pair Leprocybe-Veronicae, kept separate due to morphological differences despite a robust joint support. On the other hand, sect. Anomali, Cortinarius, and Delibuti are examples of bihemispherical, morphologically homogeneous sections with a robust support.
Merits of going beyond ITS/LSU
Comparing the 4-loci tree (Fig. 1) with the 2-loci tree (Fig. 2) one finds that they recover largely the same sections, while the bootstrap support is in many cases higher in the former. The 4-loci tree even reveals several smaller sections that were not present with two loci. It appears, in fact, that the mere addition of an rpb sequence to one sample often has a definitely positive effect on clade support (cf. Frøslev et al. 2005). Moreover, this ‘grafting’ of rpb sequences sometimes produces an extended effect, which is in retrospect perhaps not so surprising, in that it stabilises not only the ‘grafted’ clade but also neighbouring clades, even if the latter do not include the extra loci.
The use of gap coding (Nagy et al. 2012) has also proved beneficial in the present study in order to confirm many sections and increase their support.
Extent of sampling
As indicated by the number of putative section members listed (in parentheses) under Taxonomy, many species remain to be sequenced in the appropriate loci. In some cases it is especially important to provide the data to confirm (or refute) a proposed section.
Acknowledgments
We wish to thank J.F. Ammirati (University of Washington, Seattle, USA) and an anonymous reviewer for their excellent comments that were greatly beneficial for our article. J.A. Cooper and D. Park were supported through the Landcare Research Characterising Land Biota portfolio with funding from the Science and Innovation Group of the New Zealand Ministry of Business, Innovation and Employment. We are grateful to the JEC DNA group, to L.G. Nagy (BRC-HAS, Szeged, Hungary), and to G.M. Kovács (ELTE, Budapest, Hungary) for their support in the molecular study of several specimens. T.E. Brandrud, B. Buyck, G. Saar and G. Schmidt-Stohn are thanked for providing important specimens for this study.
REFERENCES
- Ammirati JF, Garnica S, Halling RE, et al. 2007. New Cortinarius species associated with Quercus and Comarostaphylis in Costa Rica. Canadian Journal of Botany 85: 794–812. [Google Scholar]
- Ammirati JF, Hughes K, Liimatainen K, et al. 2013. Cortinarius hesleri from eastern North America and related species from Europe and western North America. Botany 91: 91–98. [Google Scholar]
- Anderson TP, Orlovich DA. 2016. Cortinarius majestaticus comb. nov.: phylogenetic evidence for the transfer of Descolea majestatica to Cortinarius. Mycological Progress 15: 20. doi: 10.1007/s11557-016-1164-1. [DOI] [Google Scholar]
- Ariyawansa HA, Hyde KD, Jayasiri SC, et al. 2015. Fungal diversity notes 111–252 – taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75(1): 27–274. [Google Scholar]
- Ballarà J, Mahiques R, Garrido-Benavent I. 2017. Estudi de Cortinariaceae del Parc natural Cadí-Moixeró (IV). Moixeró 9: 20–49. [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P. 1994. Atlas des Cortinaires, Clé générale des sous-genres, sections, sous-sections et séries. Èditions Fèdération mycologique Dauphiné-Savoie, France. [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P, et al. 2001. Atlas des Cortinaires, Pars XI. Èditions Fèdération mycologique Dauphiné-Savoie, France. [Google Scholar]
- Borchsenius F. 2009. FastGap 1.2. Department of Bio-sciences, Aarhus University, Denmark: http://www.aubot.dk/FastGap_home.htm. [Google Scholar]
- Brandrud TE. 1996. Cortinarius, subgenus Phlegmacium, section Phlegmacium in Europe. A study of character variation and ecology including a numerical analysis of the C. argutus complex. Mycological Research 100(4): 471–485. [Google Scholar]
- Brandrud TE. 1998. Cortinarius subgenus Phlegmacium section Phlegmacioides (= Variecolores) in Europe. Edinburgh Journal of Botany 55(1): 65–156. [Google Scholar]
- Brandrud TE, Bellù F, Frøslev TG, et al. 2013. Cortinarius subgenus Phlegmacium section Claricolores and the story about Cortinarius blattoi Mazza. Journal des JEC 15: 14–30. [Google Scholar]
- Brandrud TE, Dima B, Schmidt-Stohn G, et al. 2014. Cortinarius subgenus Phlegmacium section Multiformes in Europe. Journal des JEC 16: 162–199. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1989. Cortinarius Flora Photographica. Vol. I (Swedish version). Cortinarius HB, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1992. Cortinarius Flora Photographica. Vol. II (Swedish version). Cortinarius HB, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1994. Cortinarius Flora Photographica. Vol. III (Swedish version). Cortinarius HB, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 1998. Cortinarius Flora Photographica. Vol. IV (Swedish version). Cortinarius HB, Sweden. [Google Scholar]
- Brandrud TE, Lindström H, Marklund H, et al. 2012. Cortinarius Flora Photographica. Vol. V (Swedish version). Cortinarius HB, Sweden. [Google Scholar]
- Brandrud TE, Schmidt-Stohn G, Liimatainen K, et al. 2018. Cortinarius sect. Riederi: taxonomy and phylogeny of the new section with European and North American distribution. Mycological Progress 17: 1323–1354. [Google Scholar]
- Dima B, Lindström H, Liimatainen K, et al. 2016. Typification of Friesian names in Cortinarius sections Anomali, Spilomei, and Bolares, and description of two new species from northern Europe. Mycological Progress 15(9): 903–919. [Google Scholar]
- Fernández-Brime S, Vila J, Ortega A. 2014. Some new and interesting taxa of Cortinarius subgenus Phlegmacium from the European Mediterranean Basin. Mycologia 106(3): 491–504. [DOI] [PubMed] [Google Scholar]
- Fries E. 1821. Systema Mycologicum. Uppsala. [Google Scholar]
- Fries E. 1851. Monographia Cortinariorum Suecicae. Uppsala. [Google Scholar]
- Frøslev TG, Brandrud TE, Jeppesen TS. 2006a. New species and combinations in Cortinarius subgenus Phlegmacium section Calochroi. Mycotaxon 97: 367–377. [Google Scholar]
- Frøslev TG, Jeppesen TS, Læssøe T, et al. 2006b. Molecular phylogenetics and delimitation of species in Cortinarius section Calochroi (Basidiomycota, Agaricales) in Europe. Molecular Phylogenetics and Evolution 44: 217–227. [DOI] [PubMed] [Google Scholar]
- Frøslev TG, Matheny PB, Hibbett DS. 2005. Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): A comparison of rpb1, rpb2, and ITS phylogenies. Molecular Phylogenetics and Evolution 37: 602–618. [DOI] [PubMed] [Google Scholar]
- Garnica S, Schön ME, Abarenkov K, et al. 2016. Determining threshold values for barcoding fungi: lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiology Ecology 92(4): fiw045. doi: 10.1093/femsec/fiw045. [DOI] [PubMed] [Google Scholar]
- Garnica S, Spahn P, Oertel B, et al. 2011. Tracking the evolutionary history of Cortinarius species in section Calochroi, with transoceanic disjunct distributions. BMC Evolutionary Biology 11: 213 http://www.biomedcentral.com/1471-2148/11/213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oberwinkler F. 2002. New Cortinarius species from Nothofagus forests in South Chile. Mycologia 94(1): 136–145. [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oberwinkler F. 2003. Morphological and molecular phylogenetic studies in South American Cortinarius species. Mycological Research 107(10): 1143–1156. [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oertel B, et al. 2005. A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Canadian Journal of Botany 83: 1457–1477. [Google Scholar]
- Garnica S, Weiß M, Oertel B, et al. 2009. Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences. BMC Evolutionary Biology 9: 1. doi: 10.1186/1471-2148-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Benavent I, Ballarà J, Mahiques R. 2014. Cortinarius cadi-aguirrei, un nou tàxon de la secció Fulvescentes Melot. Journal des JEC 16: 24–34. [Google Scholar]
- Gasparini B. 2004. Cortinarius, subgenus Orellani in Australia and in the world. Australasian Mycologist 23(2): 62–76. [Google Scholar]
- Gasparini B. 2006. Renaming of three Australian Cortinarius. Australasian Mycologist 24(3): 24–27. [Google Scholar]
- Gasparini B. 2007. Genus Cortinarius, subgenus Phlegmacium in Tasmania. New Zealand Journal of Botany 45: 155–236. [Google Scholar]
- Gasparini B. 2013. Cortinarius (Agaricales) revised taxonomy: new species names or combinations. Mycosphere 4(3): 363–454. [Google Scholar]
- Gasparini B. 2016. Cortinarius (Agaricales) revised taxonomy: validation of new species names or combinations. IOSR Journal of Pharmacy. 6(4): 7–8. [Google Scholar]
- Gasparini B, Soop K. 2008. Contribution to the knowledge of Cortinarius [Agaricales, Cortinariaceae] of Tasmania (Australia) and New Zealand. Australasian Mycologist 27(3): 173–203. [Google Scholar]
- Gill M. 1995. Invited review Pigments of Australasian dermocybe toadstools. Australian Journal of Chemistry 48(1): 1–26. [Google Scholar]
- Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27: 221–224. [DOI] [PubMed] [Google Scholar]
- Grgurinovic CA. 1997. Larger fungi of South Australia. The Botanic Gardens of Adelaide and State Herbarium and the Flora and Fauna of South Australia Handbooks Committee, Australia. [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52(5): 696–704. [DOI] [PubMed] [Google Scholar]
- Harrower E, Ammirati JF, Cappuccino A, et al. 2011. Cortinarius species diversity in British Columbia and molecular phylogenetic comparison with European specimen sequences. Botany 89: 799–810. [Google Scholar]
- Harrower E, Bougher N, Henkel T, et al. 2015a. Long-distance dispersal and speciation of Australasian and American species of Cortinarius sect. Cortinarius. Mycologia 107(4): 697–709. [DOI] [PubMed] [Google Scholar]
- Harrower E, Bougher N, Winterbottom C, et al. 2015b. New species in Cortinarius section Cortinarius (Agaricales) from the Americas and Australasia. MycoKeys 11: 1–21. [Google Scholar]
- Høiland K. 1983. Cortinarius, subgen. Dermocybe. Opera Botanica 71: 1–112. [Google Scholar]
- Høiland K, Holst-Jensen A. 2000. Cortinarius phylogeny and possible taxonomic implications of ITS rDNA sequences. Mycologia 92(4): 694–710. [Google Scholar]
- Horak E. 1983. Mycogeography in the South Pacific region: Agaricales, Boletales. Australian Journal of Botany, supplement 10: 1–41. [Google Scholar]
- Horak E, Moser M. 1965. Fungi austroamericani XII: Studien zur Gattung Thaxterogaster. Nova Hedwigia. 10(1–2): 212–249. [Google Scholar]
- Hyde KD, Hongsanan S, Jeewon R, et al. 2016. Fungal diversity notes 367–491: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80(1): 1–270. [Google Scholar]
- Jeewon R, Hyde KD. 2016. Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7(11): 1669–1677. [Google Scholar]
- Katoh K, Kuma K, Toh H, et al. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, et al. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühner R, Romagnesi H. 1953. Flore analytique des champignons supérieurs. Masson, France. [Google Scholar]
- Kytövuori I, Niskanen T, Liimatainen K, et al. 2005. Cortinarius sordidemaculatus and two new related species, C. anisatus and C. neofurvolæsus, in Fennoscandia (Basidiomycota, Agaricales). Karstenia 45: 33–49. [Google Scholar]
- Liimatainen K. 2016. Nomenclatural novelties. Index Fungorum 294: 1. [Google Scholar]
- Liimatainen K, Niskanen T, Ammirati JF, et al. 2015. Cortinarius, subgenus Telamonia, section Disjungendi, cryptic species in North America and Europe. Mycological Progress 14: 1016. doi: 10.1007/s11557-014-1016-9. [DOI] [Google Scholar]
- Liimatainen K, Niskanen T, Dima B, et al. 2014. The largest type study of Agaricales species to date: bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia 33: 98–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locquin M. 1953. Recherches sur l’organisation et le développement des Agarics, des Bolets et des Clavaires. Bulletin de la Société Mycologique de France 69(4): 389–402. [Google Scholar]
- Moser M. 1969. Cortinarius Fr. Untergattung Leprocybe subgen. nov., Die Rauköpfe. Zeitschrift für Pilzkunde 35(3): 213–248. [Google Scholar]
- Moser M. 1986. Cortinarius Fr. subgen. Cortinarius in the SW-Pacific area. Sydowia 39: 138–147. [Google Scholar]
- Moser M, Horak E. 1975. Cortinarius Fr. und nahe verwandte Gattungen in Südamerika. Beihefte zur Nova Hedwigia 52: 1–607. [Google Scholar]
- Murrill WA. 1944. More fungi from Florida. Lloydia 7(4): 303–327. [Google Scholar]
- Nagy LG, Kocsubé S, Csanádi Z, et al. 2012. Re-mind the gap! Insertion–deletion data reveal neglected phylogenetic potential of the nuclear ribosomal internal transcribed spacer (ITS) of Fungi. PLoS ONE 7(11): e49794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen T. 2008. Cortinarius subgenus Telamonia p.p. in North Europe. Doctoral thesis, Helsinki, Finland. [Google Scholar]
- Niskanen T, Kytövuori I, Liimatainen K. 2009. Cortinarius sect. Brunnei (Basi-diomycota, Agaricales) in North Europe. Mycological Research 113: 182–206. [DOI] [PubMed] [Google Scholar]
- Niskanen T, Kytövuori I, Liimatainen K. 2011. Cortinarius sect. Armillati in northern Europe. Mycologia 103(5): 1080–1101. [DOI] [PubMed] [Google Scholar]
- Niskanen T, Kytövuori I, Liimatainen K, et al. 2013. The species of Cortinarius, section Bovini, associated with conifers in northern Europe. Mycologia 105(4): 977–993. [DOI] [PubMed] [Google Scholar]
- Niskanen T, Liimatainen K, Kytövuori I, et al. 2012. New Cortinarius species from conifer-dominated forests of North America and Europe. Botany 90: 743–754. [Google Scholar]
- Niskanen T, Liimatainen K, Kytövuori I, et al. 2015. Nomenclatural novelties. Index Fungorum 256: 1–2. [Google Scholar]
- Niskanen T, Liimatainen K, Kytövuori I, et al. 2016. Cortinarius subgenus Callistei in North America and Europe – type studies, diversity, and distribution of species. Mycologia 108(5): 1018–1027. [DOI] [PubMed] [Google Scholar]
- Orlovich D, Oliver AM. 2002. The taxonomic identity of Gymnopilus rubrocastaneus recently described from New Zealand. New Zealand Journal of Botany 40: 481–487. [Google Scholar]
- Ortega A, Suárez-Santiago V, Reyes J. 2008. Morphological and ITS identification of Cortinarius species (section Calochroi) collected in Mediterranean Quercus woodlands. Fungal Diversity 29: 73–88. [Google Scholar]
- Papp V, Dima B. 2018. New systematic position of Aurantiporus alborubescens (Meruliaceae, Basidiomycota), a threatened old-growth forest polypore. Mycological Progress 17: 319–332. [Google Scholar]
- Peintner U, Bougher N, Castellano M, et al. 2001. Multiple origins of sequestrate fungi related to Cortinarius. American Journal of Botany 88(12): 2168–2179. [PubMed] [Google Scholar]
- Peintner U, Horak E, Moser M, et al. 2002. Phylogeny of Rozites, Cuphocybe and Rapacea inferred from ITS and LSU rDNA sequences. Mycologia 94(4): 620–629. [DOI] [PubMed] [Google Scholar]
- Peintner U, Moncalvo JM, Vilgalys R. 2004. Toward a better understanding of the infrageneric relationships in Cortinarius. Mycologia 96(5): 1042–1058. [PubMed] [Google Scholar]
- Saar G, Brandrud TE, Dima B, et al. 2014. Cortinarius untergattung Phlegmacium sektion Purpurascentes in Europa. Journal des JEC 16: 140–161. [Google Scholar]
- Salgado Salomón ME, Dresch P, Horak E, et al. 2018. The enigmatic Cortinarius magellanicus complex occurring in Nothofagaceae forests of the Southern Hemisphere. Fungal Biology 122(11): 1077–1097. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H, Carr TG. 2001. Incorporation, relative homoplasy, and effect of gap characters in sequence-based phylogenetic analysis. Systematic Biology 50(3): 454–462. [PubMed] [Google Scholar]
- Singer R. 1986. Agaricales in modern taxonomy. Koeltz, Königstein. [Google Scholar]
- Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution 12: 335–337. [Google Scholar]
- Smith AH. 1966. Notes on Dendrogaster, Gymnoglossum, Protoglossum, and species of Hymenogaster. Mycologia 58(1): 106–112. [Google Scholar]
- Soop K. 1998. Notes et observations sur les champignons cortinarioïdes de Nouvelle-Zélande. Documents Mycologiques 112: 13–26. [Google Scholar]
- Soop K. 2001. Contribution à l’étude de la mycoflore cortinarioïde de Nouvelle-Zélande. Bulletin de la Société Mycologique de France 117(2): 91–132. [Google Scholar]
- Soop K. 2002. Contribution à l’étude de la mycoflore cortinarioïde de Nou-velle-Zélande II. Bulletin de la Société Mycologique de France 118(3):173–194. [Google Scholar]
- Soop K. 2005. A contribution to the study of the cortinarioid mycoflora of New Zealand IV. New Zealand Journal of Botany 43: 551–562. [Google Scholar]
- Soop K. 2016. A contribution to the study of the cortinarioid mycoflora of New Zealand VII. New Zealand Journal of Botany 54(3): 344–365. [Google Scholar]
- Soop K, Dima B, Szarkándi JG, et al. 2016. Psathyloma, a new genus in Hymenogastraceae described from New Zealand. Mycologia 108(2): 397–404. [DOI] [PubMed] [Google Scholar]
- Soop K, Gasparini B. 2011. Europe and the South Pacific: A comparison of two Cortinarius floras. Journal des JEC 13: 99–106. [Google Scholar]
- Soop K, Wallace M, Dima B. 2018. New Cortinarius (Agaricales) species described from New Zealand. New Zealand Journal of Botany 56(2): 163–182. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani F, Jones RH, May TW. 2013. Concordance of seven gene genealogies compared to phenotypic data reveals multiple cryptic species in Australian dermocyboid Cortinarius (Agaricales). Molecular Phylogenetics and Evolution 71: 249–260. [DOI] [PubMed] [Google Scholar]
- Stensrud Ø, Orr R, Reier-Røberg K. 2014. Phylogenetic relationships in Cortinarius with focus on North European species. Karstenia 54: 57–71. [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28(10): 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution 30(12): 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Qiu YL. 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16: 299–363. [DOI] [PubMed] [Google Scholar]
- Wilson A, May T, Mueller G. 2017. Biogeography of the ectomycorrhizal mushroom genus Laccaria. In: Tedersoo L. (ed) Biogeography of Mycorrhizal Symbiosis, Ecological Studies 230: 273–297. [Google Scholar]


