Abstract
Triclosan (TCS) is an antibacterial widely used in personal care products that exhibits endocrine disrupting activity in several species, with reports of altered thyroid, estrogen and androgen signaling pathways. To evaluate the androgenic mode of action, TCS was evaluated for androgen receptor mediated effects in the Hershberger assay and for altered androgen synthesis in the H295R steroidogenesis assay. In the Hershberger assay, castrated males were dosed by oral gavage for 10 days with corn oil (vehicle) or TCS (50 or 200 mg/kg/day) in the presence or absence of testosterone proprionate (TP, 0.2 mg/kg/day) prior to assessing accessory sex tissues (ASTs) weights. TCS alone or in combination with TP did not alter androgen dependent AST weights. Assessment of serum thyroxine (T4) demonstrated a significant dose-dependent decrease by TCS (50 or 200 mg/kg/day) co-administered with TP and TCS (200 mg/kg) without TP, but no differences in liver or thyroid weights. In the H295R assay, TCS from 0.01 to 10 μM had no effect on testosterone production but TCS at 3 μM and above did induce a significant increase in estrogen production. At 10 μM, TCS produced significant cytotoxicity which confounded the interpretation of the estrogenic effect at that concentration. Thus, TCS had no effect on androgen synthesis or activity in the models used, but did enhance estrogen production and suppress serum T4.
Keywords: Triclosan, Hershberger, H295R, Steroidogenesis, Endocrine disruptor
1. Introduction
Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol, TCS) is an antimicrobial compound that has been commonly found in various personal care products since the 1960s, including deodorant, toothpaste, soaps, and cosmetics (Witorsch, 2014). Recently, the FDA limited the use of TCS in household body washes and soaps (FDA, 2016). Prior to that new ruling, it was detected in urban effluent waters at 37.8 μg/L and surface waters at 431 ng/L (Hua et al., 2005; Kolpin et al., 2002; Morrall et al., 2004). Furthermore, TCS has been and detected in human body fluids including blood plasma, urine, and breast milk (Calafat et al., 2008; Dayan, 2007, Hovander et al., 2002).
The role of TCS as an endocrine disruptor has been investigated both in vivo and in vitro to discern its effects on male reproduction and thyroid function. In the environment, TCS bioaccumulates and has exhibited endocrine disrupting effects in fish and amphibians (Adolfsson-Erici et al., 2002; Ishibashi et al., 2004; Veldhoen et al., 2006). TCS may have weak androgenic activity in fish as demonstrated by changes in fin length, sex ratios, and hatchability of fertilized eggs (Foran et al., 2000; Ishibashi et al., 2004). TCS exhibits estrogenic properties indicated by increased vitellogenin in male medaka fish exposed to 20–100 μg/L of TCS (Ishibashi et al., 2004). In male rats, conflicting studies report that TCS disrupted steroidogenic production of testosterone (T) and decreased accessory sex tissue (AST) weights, while others found no changes in pubertal timing or the growth of ASTs after peripubertal exposure (Kumar et al., 2009; Zorrilla et al., 2009).
In vitro studies examining the possible androgenic activity of TCS include several androgen receptor (AR) reporter cell-based assays using transgenic human embryonic kidney cells, transfected murine mammary tumor cells, or a bioluminescent yeast-based assay (Chen et al., 2007; Gee et al., 2008; Svobodova et al., 2009). While these studies were unable to demonstrate androgenic activity of TCS through agonist activation of AR, Gee et al., 2008, demonstrated an anti-androgenic effect of TCS in a receptor binding assay and a mouse mammary tumor AR reporter gene assay at concentrations of 0.1 μM–100 μM. Another study demonstrated a similar effect, with suppression of testosterone transcriptional activation in HEK293 cells transfected with the human AR at TCS concentrations of 1 and 10 μM (Chen et al., 2007). TCS may also stimulate co-factors or co-activators in AR containing cells as indicated by the TCS effects on MDA-kb2 cells where TCS (0.01–1uM) enhanced dihydrotestosterone (0.5 nM) activation of the AR (Christen et al., 2010).
TCS exposure has also been shown to result in changes in estrogen dependent processes, such as advancing puberty and enhancing the estrogen response in the uterus (Louis et al., 2013; Stoker et al., 2010). However, the in vitro effects of TCS on steroid receptor binding or steroidogenesis are disparate. For example, TCS inhibited cell growth induced by 17ß estradiol in MCF7 human breast cancer cells, indicating TCS may have anti-estrogenic effects (Gee et al., 2008), while our laboratory found that TCS did not significantly alter estrogen receptor (ER) transcriptional activation in T47D-KDBluc breast cancer cells (Louis et al., 2013).
In rats, TCS has demonstrated the ability to decrease serum thyroid hormone (T4) regardless of sex, strain, or age at exposure (Axelstad et al., 2013; Crofton et al., 2007; Paul et al., 2012; Paul et al., 2010b; Stoker et al., 2010; Zorrilla et al., 2009). In addition, TCS increases the catabolic elimination of T4 by increasing the expression of Phase I and II hepatic enzymes involved in thyroid hormone metabolism (Paul et al., 2010b).
To further investigate the effects of TCS on androgenic activity, TCS was examined in both the Endocrine Disruptors Screening Program (EDSP) Tier 1 male rat Hershberger assay with additional serum T4 measurement and the in vitro H295R steroidogenesis assay. In the Hershberger study, animals were treated with testosterone proprionate (TP) alone or in combination with TCS to evaluate the possible effects of TCS on AR-mediated endpoints, as well as additional endpoints for evaluating the thyroid pathway. To evaluate the effects of TCS on steroidogenesis, testosterone (T) and estrogen (E) production were assessed after a 48-h exposure to TCS in the human adrenocortical carcinoma H295R cell line.
2. Materials & methods
2.1. Test chemicals
Triclosan (99.5%), forskolin (98%), testosterone proprionate and DMSO (99.9%) were obtained from Sigma Chemical Co. (St. Louis, MO). Prochloraz (99.5%) was obtained from Chem Services Inc. (West Chester, PA). For the H295R assay, chemicals were tested at seven different concentrations (0.01–30 μM) in triplicate per exposure plate. A quality control (QC) plate included with each run contained a blank control, solvent control (DMSO), the inhibitor prochloraz (0.3 and 3 μM), and the stimulator forskolin (1 and 10 μM).
2.2. Hershberger assay
TCS was tested for its anti-androgenicity by administration to castrated, testosterone-treated rats. This in vivo assay detects anti-androgenic activity simply by weighing androgen-dependent accessory sex tissues (ASTs) following treatment in the castrated male rat (Gray et al., 2004; Hershberger et al., 1953). Male Wistar rats were obtained from Charles Rivers Laboratories (Raleigh, NC) at weaning and housed three per cage (clear polycarbonate cages) with heat-treated laboratory grade pine shavings (to eliminate resins that induce liver enzymes). Environmental conditions were maintained on a 14:10-h light schedule in an AAALAC accredited facility (temperature 20–22° C and a humidity of 40–50%) and provided with Purina 5001 rat chow and filtered (5 μm) municipal water ad libitum. Males were castrated under isoflurane anesthesia at 41 days of age and allowed to acclimate for 10 days and then weighed, weight ranked, and equally distributed amongst the treatment groups at 51 days of age. Doses of triclosan were selected based on our previous 30-day exposure to the prepubertal male rat (Zorrilla et al., 2009). In this study, each rat was dosed by oral gavage (2.5 mL/kg body weight) daily with TCS (50 or 200 mg/kg) in corn oil alone or in combination with subcutaneous injection of 0.2 mg/kg testosterone proprionate (TP) from postnatal day 53 to PND 62 (6 rats per treatment group). Controls received corn oil only. All rats were treated at approximately 0800 h. After decapitation on PND 63, ASTs were removed and weighed, including the ventral prostate, Cowper’s glands, seminal vesicle (with coagulating glands and fluids), glans penis and levator ani plus bulbocavernosus muscles. The liver and thyroids were also weighed at the necropsy. Blood collected at decapitation was allowed to clot for 1 h and then centrifuged at 1260_g for 30 min, the serum collected and stored frozen at −80° C for subsequent hormone assays (T and T4). The serum was thawed and levels of Total testosterone and Total T4 were measured using Coat-a-Count® radioimmunoassay (RIA) kits obtained from Diagnostic Products Corporation (Los Angeles, CA).
2.3. H295R steroidogenesis assay
H295R cells are derived from a pluripotent adrenocortical carcinoma cell line and are used in a cell bioassay to evaluate the effects of chemicals on steroidogenesis, namely the production of testosterone and estradiol. Cells were obtained from the American Type Culture Collection (ATCC #CRL-2128; Manassas, VA) and cultured as described previously (Hecker et al., 2007). Briefly, cells were grown in 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mix (DMEM/F12) containing 1.2 g/L Na2CO3, 5 mL/L ITS+ Premix (BD Biosciences), and 12.5 mL/L Nu-Serum (BD Biosciences). Cells were cultured in 75 cm2 flasks at 37 °C with a 5% CO2 atmosphere. Cells were seeded 1 mL/well in 24-well cell culture plates at a density of 3×105 cells/ml. Cells were allowed a 24 h acclimation and attachment period and then medium was exchanged and exposure to TCS (0.01, 0.1, 0.3, 1, 3, or 10 μM), prochloraz (0.3 and 3 μM), and forskolin (1.0 and 10 μM) was initiated. DMSO was used as carrier solvent and did not exceed 0.1% v/v. After 48 h of chemical treatment, the medium was removed and stored at −80° C until hormone analysis was performed. Media levels of Total testosterone and Total estradiol were measured using Coat-a-Count® RIA-kits obtained from Diagnostic Products Corporation (Los Angeles, CA; lowest detectable levels were 0.04 ng/ml and 8.0 ng/ml, respectively). Immediately following termination of the chemical exposure, cell viability was evaluated in the same plates with a Live/Dead ® Viability/Cytotoxicity Kit (Invitrogen). Briefly, after washing with PBS, cells were stained with calcein AM and ethidium homodimer-1 (EthD-1) for 1 h. Fluorescence was measured using a Wallac®1420D fluorometer single plate reader (PerkinElmer). Excitation and emission filters were 485 nm and 515 nm for calcein and 485 nm and 635 nm for EthD-1. The percentage of live or dead cells was calculated from the fluorescence readings in sample wells in relation to reference wells where all cells are alive or dead (70% methanol) according to the formulae provided by the manufacturer.
2.4. Aromatase activity assay
Direct effects of TCS on aromatase (CYP19A) activity were assessed by a cell-free kit obtained from Biovision Incorporated (Milpitas, CA). All reactions in the assay were performed in duplicate. The effect of TCS on aromatase activity was evaluated at 1, 3, 10, and 30 μM. In addition, aromatase activity alone or in the presence of a positive inhibitor, Letrozole (5 μM), was also assessed in the same assay. Human recombinant aromatase and Letrozole were provided by the manufacturer. The aromatase was incubated with the reaction mix, assay buffer, and chemical treatments for 10 min at 37° C. Aromatase substrate was then added to each well and immediately read on a FLUOstar Omega fluorescence microplate reader (BMG Labtech, Ortenberg, Germany). Excitation and emission filters were set at 485 nm and 527 nm and readings collected every 10 min. The specific activity of aromatase (pmole/min) was calculated from the fluorescence readings in sample wells in relation to reference wells of the standard curve according to the formulae provided by the manufacturer.
2.5. Statistical analyses
Hormone and tissue weight data were analyzed for treatment effects by a One-Way ANOVA and for homogeneity of variance using Bartlett’s test using the PRISM, GraphPad 6 software (GraphPad Software Inc, La Jolla, CA). When statistically significant (p< 0.05) treatment effects were indicated, the Dunnett’s t-test was used to compare each treatment group with the appropriate control. A Fisher’s Exact test was used to analyze the Live/Dead assay cytotoxicity data.
3. Results
3.1. Hershberger assay
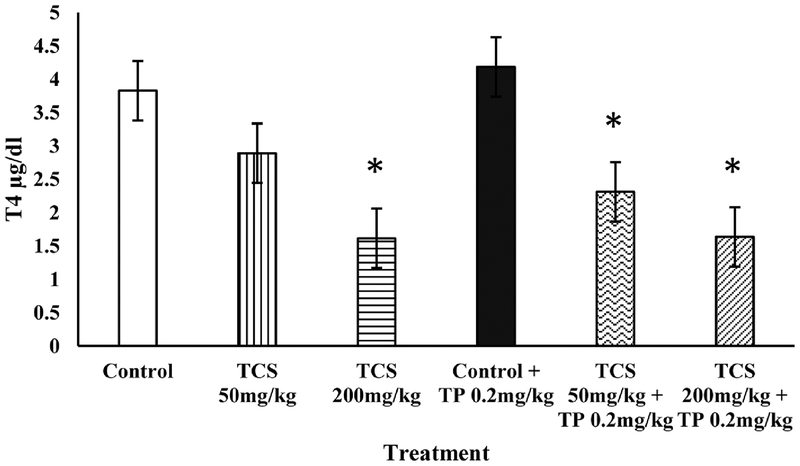
The Hershberger assay was used to elucidate the androgenic or antiandrogenic effects of TCS. Body weights following the exposure period were not different from mean control body weights in any treatment group (Table 1). TCS did not induce growth of any of the AST when administered alone or in combination with TP. In addition, there was no difference in mean liver or thyroid weight as compared to controls. However, TCS significantly decreased T4 at 200 mg/kg males with and without TP; while males with TP showed a significant decrease at both 50 and 200 mg/kg TCS (Fig. 1).
Table 1.
Effect of Triclosan on mean (+/− SEM) organ/accessory sex tissue weights of castrated male rats in the Hershberger assay.
| Treatment | BW (g) | GP (mg) | SV (mg) | VP (mg) | CG (mg) | LABC (mg) | Liver (g) | Thyroid (mg) | N |
|---|---|---|---|---|---|---|---|---|---|
| Control+(sc) Oil | 311 ± 8.11 | 44.9 ± 1.73 | 36.9 ± 3.79 | 16.0 ± 0.22 | 4.53 ± 0.57 | 166 ± 8.84 | 13.8 ± 1.52 | 19.8 ± 2.47 | 5 |
| Control+(sc) TP 0.2mg/kg | 317 ± 12.3 | 74.1 ± 3.40 | 173 ± 14.0 | 70.3 ± 7.85 | 21.9 ± 2.11 | 325 ± 22.1 | 14.2 ± 0.84 | 22.4 ± 1.30 | 7 |
| TCS 50 mg/kg+(sc) Oil | 300 ± 9.40 | 45.6 ± 1.87 | 30.6 ± 5.45 | 13.5 ± 1.72 | 4.98 ± 1.05 | 153 ± 9.03 | 12.3 ± 0.34 | 18.6 ± 1.15 | 5 |
| TCS 50 mg/kg+(sc) TP 0.2 mg/kg | 315 ± 5.67 | 74.2 ± 2.97 | 206 ± 12.8 | 74.2 ± 4.01 | 24.0 ± 2.48 | 307 ± 9.90 | 15.8 ± 0.47 | 20.0 ± 1.21 | 6 |
| TCS 200 mg/kg+(sc) Oil | 308 ± 5.53 | 45.9 ± 3.78 | 29.3 ± 0.73 | 13.6 ± 1.04 | 4.86 ± 0.84 | 153 ± 9.30 | 14.8 ± 0.90 | 20.4 ± 0.90 | 5 |
| TCS 200 mg/kg+(sc) TP 0.2 mg/kg | 315 ± 12.5 | 72.0 ± 1.91 | 180 ± 11.1 | 86.1 ± 7.17 | 25.4 ± 2.36 | 318 ± 18.2 | 15.0 ± 0.59 | 21.6 ± 1.53 | 6 |
Note: sc=subcutaneous, TP=testosterone proprionate, BW=body weight, GP=glans penis, SV=seminal vesicle, CG=coagulating gland, LABC=levitor ani plus bulbocavernosus muscle. None of the treatment mean weights were significantly different from the corresponding control (oil or TP).
Fig. 1.
Total serum T4 (μg/dl) concentrations in castrated male rats treated with TCS in the presence or absence of testosterone proprionate (TP; 0.2 mg/kg). Bars represent mean ± SEM. *P < 0.01 as compared to corresponding control (white or black bar).
3.2. H295R steroidogenesis assay
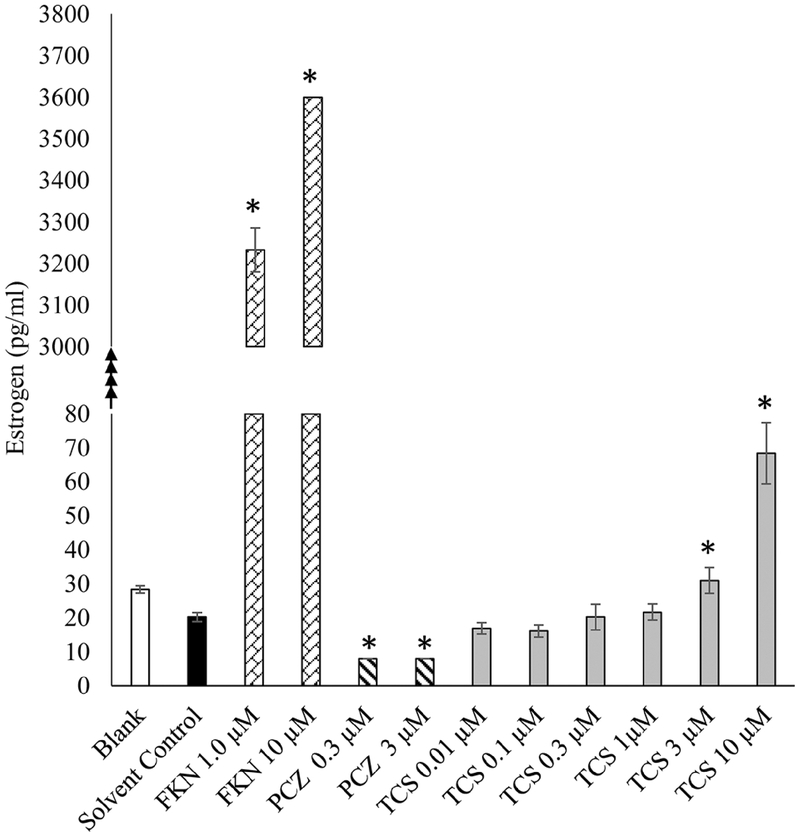
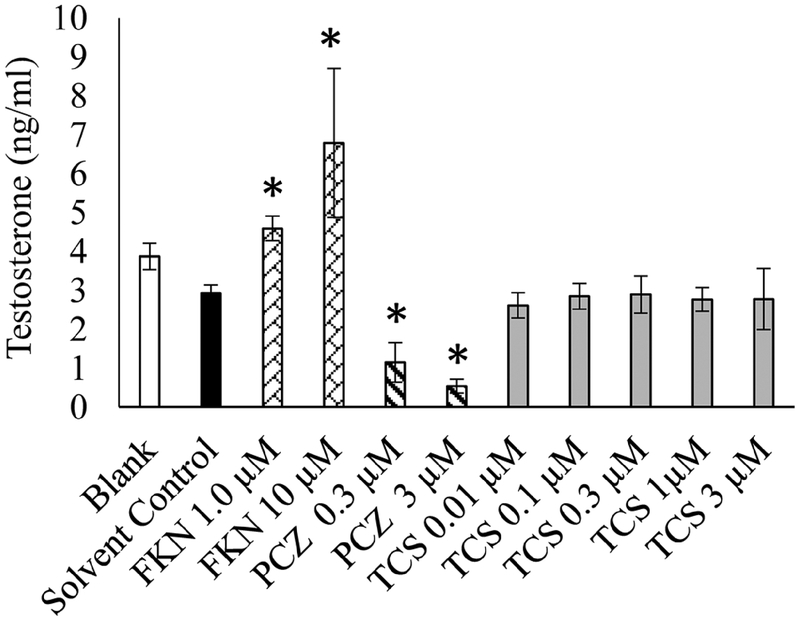
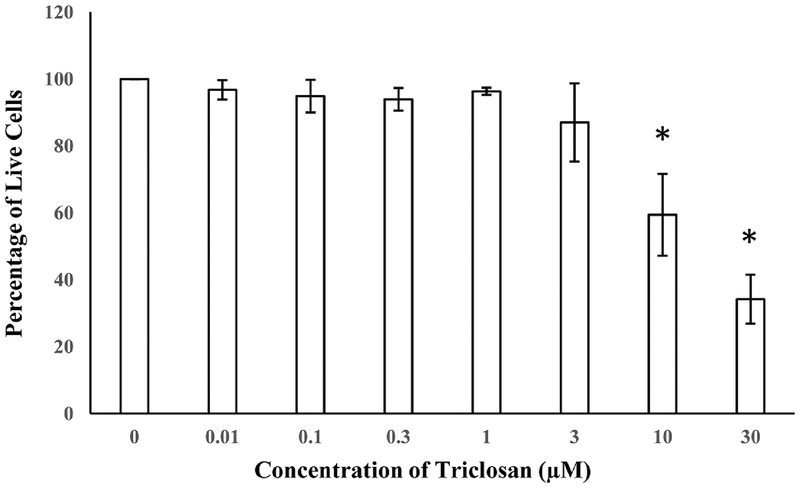
The EDSP Tier 1 H295R in vitro assay was used to examine the effects of TCS on E and T production. In this assay, cellular production of E and T were appropriately increased or decreased after exposure to Forskolin (positive stimulatory control) or Prochloraz (positive inhibitory control), respectively. Cells exposed to 3 μM TCS and above exhibited a significant dose dependent increase in E production compared to DMSO 0.1% controls (Fig. 2). In contrast, TCS did not exhibit a significant change in T as compared to basal secretion in the DMSO controls (Fig. 3). Live/dead assay revealed significant cytotoxicity in cells exposed to 10 μM and 30 μM TCS, reducing the number of viable cells by 40% and 74%, respectively (Fig. 4). However, cell viability at 3 μM TCS and below was not significantly different from controls.
Fig. 2.
Effect of Triclosan on production of estradiol in H295R cells in vitro. Black bar indicates cells treated with solvent control only (DMSO 0.1%). Forskolin (FKN), positive control for induced estradiol production. Prochloraz (PCA), positive control for inhibition of steroid production. Gray bars indicate cells treated with Triclosan in μM concentration. *P < 0.05 compared to solvent control.
Fig. 3.
Effect of Triclosan on production of testosterone in H295R cells in vitro. Black Bar indicates cells treated with only DMSO 0.1%. Forskolin (FKN), positive control for induced testosterone production. Prochloraz (PCZ), positive control for inhibition of T production. Gray bars indicate cells treated with Triclosan in μM concentration. *P < 0.05 compared to solvent control.
Fig. 4.
Cell viability of H295R cells after 48 h exposure to TCS from 0.01 to 30 μM (n=6). * P < 0.05 as compared to control (DMSO 0.1%).
3.3. Aromatase activity assay
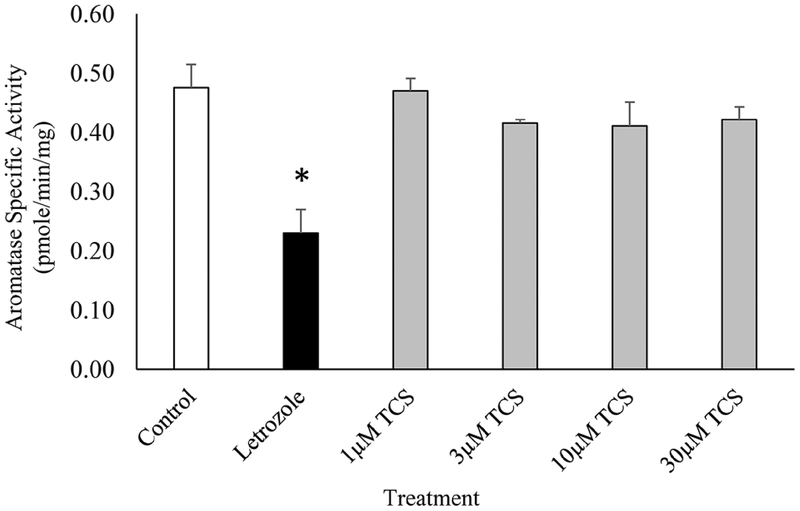
The effect of TCS on aromatase activity was assessed in a fluorometric cell-free assay following a 10 min exposure. Letrozole, the positive control, significantly inhibited the human recombinant aromatase specific activity by 50%, indicating good assay performance. However, aromatase activity was unaffected by TCS exposure at 1, 3, 10, or 30 μM as compared to the aromatase control (Fig. 5).
Fig. 5.
The effect of Triclosan on human recombinant aromatase activity. White bar indicates control (Aromatase alone) (n=4), Black bar indicates Aromatase plus 5 μM Letrozole (n=3). Gray bars indicate Aromatase plus Triclosan at 1, 3, 10, or 30 μM (n=4, 4, 5, or 5 respectively). * P < 0.05 as compared to control.
4. Discussion
In the Hershberger EDSP Tier 1 assay, TCS did not significantly alter the weight of any of the androgen dependent ASTs, indicating that TCS did not agonize or antagonize the androgen receptor (AR) in the rat (Table 1). These results support our previous findings (Zorrilla et al., 2009). Prepubertal male Wistar rats were dosed for 31 days from PND23 through puberty to PND 53 with 3 to 300 mg/kg/day of TCS. TCS had no effect on pubertal onset or AST weights, and did not change levels of serum or pituitary luteinizing hormone (LH) and prolactin, or serum androstenedione. Serum T was not different in animals treated with 3, 30, 100, or 300 mg/kg/day of TCS, however, a decrease of 60% was observed in males treated with 200 mg/kg/day of TCS in one block. A more recent study also found no effect on male reproductive endpoints such as anogenital distance, nipple retention or prostate weights in male offspring of Wistar dams dosed with 75 to 300 mg/kg/day of TCS from GD7 to GD21 whose pups were subsequently dosed directly until PND16 (Axelstad et al., 2013). Combined, these studies and our current study indicate that TCS does not alter mammalian AR signaling.
In contrast, Kumar et al., 2009 dosed adult male Wistar rats with 5–20 mg/kg/day TCS for 60 days and found dose-dependent decreases in weights of the ASTs, decreased mRNA levels for steroidogenic enzymes, and significant reductions in serum LH, follicle stimulating hormone (FSH), and T. However, that study was conducted with adult rats with lower mean body weights and TCS from a different source and lower purity than the current study that may have contributed to these differences (Witorsch, 2014).
The level of testosterone produced in H295R cells over the 48 h exposure period was unaffected by any TCS concentration (Fig. 3). Other studies using rat Leydig cells to examine effects of TCS on steroidogenesis found that TCS inhibited LH-induced T synthesis in a dose dependent manner from 0.01 to 10 μM without cytotoxicity (Kumar et al., 2008). The differences in T synthesis and cytotoxicity between these studies are most likely attributable to possible contaminants in the less pure TCS, differences in cell lines and the LH stimulated induction of T. In a different study, TCS (30 μM) inhibited T synthesis in human chorionic gonadotropin (hCG)-induced murine Leydig cells, however, basal T production was unaffected (Forgacs et al., 2012). H295R cells lack the LH receptor and cannot be stimulated by LH to produce T, indicating that TCS may alter T production through the LH pathway. However, we found no effect in the male pubertal rat assay which argues against this possibility (Zorrilla et al., 2009).
TCS significantly increased E production in the H295R assay, at concentrations of 3 and 10 μM TCS compared to the DMSO controls (Fig. 2), albeit the 10 μM group had significant cytotoxicity. The mechanism by which TCS may alter steroidogenesis at 3 μM is still uncertain. The increase of E could be attributed to an inductive effect of TCS on aromatase activity, with increased conversion of T to E. However, results of the aromatase activity assay (using human recombinant aromatase) indicate that TCS does not affect aromatase activity, indicating that TCS is likely stimulating E production through a different mechanism. In an earlier study in our laboratory, we observed effects in agreement with this increased E production, including advancement of puberty and earlier first estrus in the rat following exposure to 150 mg/kg TCS (Stoker et al., 2010). In that study, these changes were not correlated with body weight, as the mean body weight at vaginal opening was not different from controls. Therefore, our current in vitro assay results agree with previously observed female pubertal effects indicating that TCS may increase estrogen through other mechanisms of action, such as previously described effects of TCS on inhibition of estrogen sulfotransferases (James et al., 2010; Stoker et al., 2010; Louis et al., 2013).
Additional information was collected for thyroid endpoints in the Hershberger study, including T4, thyroid weight and liver weight as previously described (Stoker et al., 2005; Yamada et al., 2004). We did not observe any changes in liver weight in this study, but we have previously seen an increase in liver weight at similar doses in the pubertal rat assays (Stoker et al., 2010; Zorrilla et al., 2009) due to induction of hepatic enzymes. We did not observe a change in thyroid weight following exposure to TCS, similar to other studies (Louis et al., 2017; Paul et al., 2012; Stoker et al., 2010; Zorrilla et al., 2009). However, we did observe significant decreases in T4 (P < 0.05) in castrated males after 10 days of treatment with either TCS (200 mg/kg/day) or TCS with TP (50 and 200 mg/kg/day). The lack of significant effect of TCS on the level of serum T4 between TP treated and untreated males at 50 mg/kg may indicate an effect of androgens on T4 binding proteins affecting the level of T4 in circulation (Tahboub and Arafah, 2009). Our findings are in agreement with several in vivo studies demonstrating a decrease in T4 after TCS treatment in both sexes at different developmental stages (Axelstad et al., 2013; Crofton et al., 2007; Paul et al., 2012; Paul et al., 2010a,b; Rodriguez and Sanchez, 2010; Stoker et al., 2010; Zorrilla et al., 2009).
In conclusion, the current study confirmed that TCS does not affect androgen dependent growth of ASTs in the Hershberger assay, which agrees with our previous study showing no effects on pubertal development in the male rat. Further, we confirmed that TCS does not alter testosterone synthesis in the H295R assay. However, we did find a significant increase in E production with TCS in the H295R assay, which is in agreement with our earlier observation that female puberty was advanced following oral exposure to TCS. In addition, this study reaffirms that TCS suppresses serum T4 as shown in numerous in vivo studies. Therefore, TCS appears to have endocrine disrupting activity on thyroid and E pathways, but not androgen pathways in these mammalian models. Additional studies are needed to determine how these endocrine disrupting effects may influence human health and wildlife.
Acknowledgments
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J, 2002. Triclosan: a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 46, 1485–1489. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Boberg J, Vinggaard AM, Christiansen S, Hass U, 2013. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem. Toxicol 59, 534–540. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ. Health Perspect. 116, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL, 2007. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharmacol 221, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen V, Crettaz P, Oberli-Schrammli A, Fent K, 2010. Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere 81, 1245–1252. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Paul KB, Devito MJ, Hedge JM, 2007. Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol 24, 194–197. [DOI] [PubMed] [Google Scholar]
- Dayan AD, 2007. Risk assessment of triclosan [Irgasan] in human breast milk. Food Chem. Toxicol 45, 125–129. [DOI] [PubMed] [Google Scholar]
- FDA, 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-The-Counter Human Use. Food and Drug Administration Health and Human Services. [PubMed] [Google Scholar]
- Foran CM, Bennett ER, Benson WH, 2000. Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar. Environ. Res 50, 153–156. [DOI] [PubMed] [Google Scholar]
- Forgacs AL, Ding Q, Jaremba RG, Huhtaniemi IT, Rahman NA, Zacharewski TR, 2012. BLTK1 murine Leydig cells: a novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants. Toxicol. Sci 127, 391–402. [DOI] [PubMed] [Google Scholar]
- Gee RH, Charles A, Taylor N, Darbre PD, 2008. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J. Appl. Toxicol 28, 78–91. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Wilson V, Noriega N, Lambright C, Furr J, et al. , 2004. Use of the laboratory rat as a model in endocrine disruptor screening and testing. ILAR J. 45, 425–437. [DOI] [PubMed] [Google Scholar]
- Hershberger LG, Shipley EG, Meyer RK, 1953. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med 83, 175–180. [DOI] [PubMed] [Google Scholar]
- Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, et al. , 2002. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch. Environ. Contam. Toxicol 42, 105–117. [DOI] [PubMed] [Google Scholar]
- Hua W, Bennett ER, Letcher RJ, 2005. Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography-electrospray-tandem quadrupole mass spectrometry. Environ. Int 31, 621–630. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, et al. , 2004. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol 67, 167–179. [DOI] [PubMed] [Google Scholar]
- James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE, 2010. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ. Int 36, 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, et al. , 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams: 1999–2000: a national reconnaissance. Environ. Sci. Technol 36, 1202–1211. [DOI] [PubMed] [Google Scholar]
- Kumar V, Balomajumder C, Roy P, 2008. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology 250, 124–131. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chakraborty A, Kural MR, Roy P, 2009. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol 27, 177–185. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Stoker TE, 2013. The effect of triclosan on the uterotrophic response to extended doses of ethinyl estradiol in the weanling rat. Reprod. Toxicol 36, 71–77. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE, 2017. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J. Toxicol. Environ. Health A 80, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrall D, McAvoy D, Schatowitz B, Inauen J, Jacob M, et al. , 2004. A field study of triclosan loss rates in river water (Cibolo Creek, TX). Chemosphere 54, 653–660. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Devito MJ, Crofton KM, 2010a. Developmental triclosan exposure decreases maternal and neonatal thyroxine in rats. Environ. Toxicol. Chem 29, 2840–2844. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM, 2010b. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol. Sci 113, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, et al. , 2012. Developmental triclosan exposure decreases maternal fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology 300, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PE, Sanchez MS, 2010. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J. Toxicol. Environ. Health A 73, 1678–1688. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE, 2005. In vivo and in vitro anti-androgenic effects of DE-71: a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol. Appl. Pharmacol 207, 78–88. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM, 2010. Triclosan exposure modulates estrogen dependent responses in the female wistar rat. Toxicol. Sci 117, 45–53. [DOI] [PubMed] [Google Scholar]
- Svobodova K, Plackova M, Novotna V, Cajthaml T, 2009. Estrogenic and androgenic activity of PCBs: their chlorinated metabolites and other endocrine disruptors estimated with two in vitro yeast assays. Sci. Total Environ. 407, 5921–5925. [DOI] [PubMed] [Google Scholar]
- Tahboub R, Arafah BM, 2009. Sex steroids and the thyroid. Best practice & research. Clin. Endocrinol. Metab 23, 769–780. [DOI] [PubMed] [Google Scholar]
- Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, et al. , 2006. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat. Toxicol 80, 217–227. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ, 2014. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit. Rev. Toxicol 44, 535–555. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kunimatsu T, Miyata K, Yabushita S, Sukata T, et al. , 2004. Enhanced rat Hershberger assay appears reliable for detection of not only (anti-)androgenic chemicals but also thyroid hormone modulators. Toxicol. Sci 79, 64–74. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, et al. , 2009. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol. Sci 107, 56–64. [DOI] [PubMed] [Google Scholar]