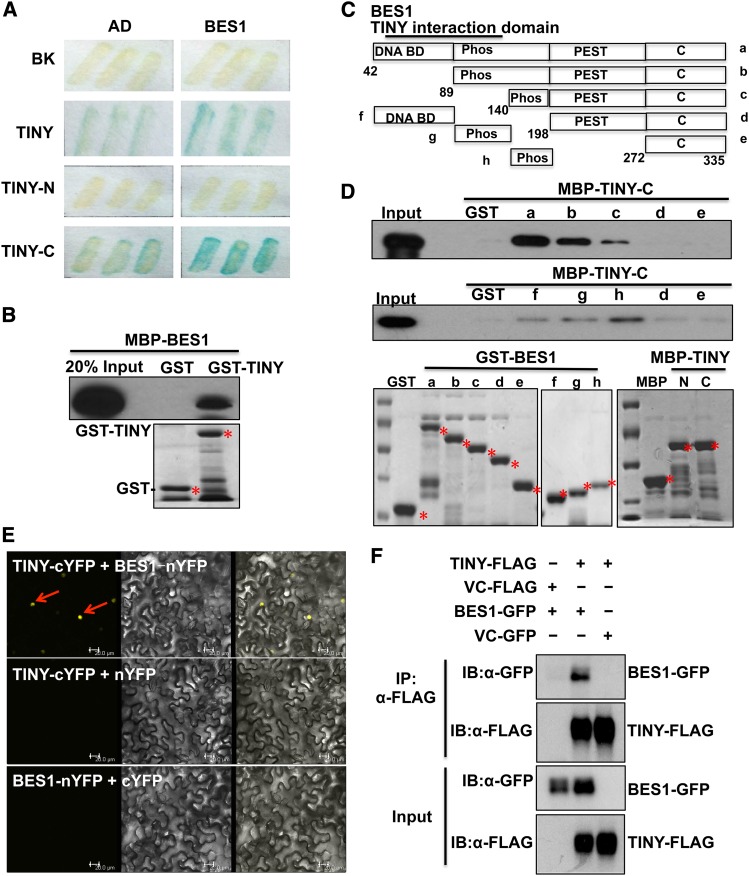
Figure 5.
TINY Interacts with BES1.
(A) TINY interacted with BES1 in yeast as detected by β-galactosidase (LacZ) activity. TINY N-terminal DNA binding domain (TINY-N) and its C-terminal activation domain (TINY-C) indicate TINY N-terminal DNA binding domain and C-terminal activation domain (AD), respectively.
(B) TINY interacted with BES1 in GST pull-down assay. Approximately equal amounts of GST, GST-TINY, and MBP-BES1 were used in the assays, as shown by a Coomassie-stained gel (bottom). Asterisks indicated the desired protein. BES1 was detected by immunoblotting using anti-MBP antibody.
(C) Schematic diagram of BES1 used for GST pull-down assay. DNA DB indicated BES1 DNA binding domain; Phos represented the phosphorylation domain of BES1; PEST represented protein sequence enriched in proline, glutamic acid, Ser, and Thr. C indicated BES1 C-terminus. (D) TINY interacted with BES1 in GST pull-down assay. Approximately equal amounts of proteins were used in the assays, as shown by a Coomassie-stained gel (bottom). TINY was detected by immunoblotting with anti-MBP antibody. Asterisks showed the desired protein.
(E) TINY interacted with BES1 by BiFC. Cotransfection of TINY-cYFP and BES1-nYFP led to the reconstitution of YFP activity in N. benthamina, whereas coexpression of TINY-cYFP and nYFP or BES1-nYFP and cYFP did not produce any positive YFP signal.
(F) Co-IP assay showed TINY and BES1 interaction. TINY-FLAG and BES1-GFP as well as control vectors were cotransformed into Arabidopsis protoplasts overnight. After 20 μM MG132 treatment, protoplasts were collected and protein was immunoprecipitated with anti-FLAG and detected with anti-FLAG and anti-GFP antibodies.