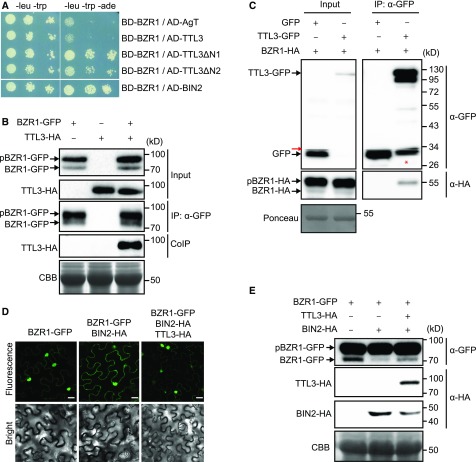
Figure 6.
TTL3 Interacts with BZR1 and Regulates Its Cytoplasmic/Nuclear Localization.
(A) Yeast-two-hybrid assays to determine the interaction of BZR1 with TTL3, the TTL3 fragment TTL3ΔN1 (amino acids 204 to 691), the TTL3 fragment TTL3ΔN2 (amino acids 371 to 691), and BIN2. Interaction of BZR1 with a fragment of Simian virus 40 large T-antigen (AD-AgT) was also included to show BD-BZR1 self-activation capacity. Growth on plasmid-selective media (left column) and interaction-selective media (lacking adenine, -ade], right column) are shown.
(B) TTL3 co-immunoprecipitates with BZR1. TTL3-HA and BZR1-GFP were transiently expressed in N. benthamiana. BZR1-GFP was IP with anti-GFP Trap beads. Total (input), IP, and CoIP proteins were analyzed by immunoblotting. Equal loading was confirmed by Coomassie blue staining (CBB) of input samples. BZR1-GFP and TTL3-HA were detected with anti-GFP and anti-HA, respectively. The top band corresponds to phosphorylated BZR1 (pBZR1-GFP) and the bottom band to dephosphorylated BZR1 (BZR1-GFP).
(C) Co-IP of BZR1-HA with TTL3-GFP expressed in transfected Arabidopsis Col-0 protoplasts. Samples were analyzed as in (B). Protoplasts cotransfected with free GFP and BRI1-HA, were used as a negative control for Co-IP. Equal loading was confirmed by Ponceau staining of input samples. TTL3-GFP and free GFP were detected with anti-GFP antibody and BRI1-HA was detected with anti-HA antibody. Asterisk indicates GFP that results from proteolytic cleavage of TTL3-GFP. Red arrow indicates an artifact from imaging the blot with high sensitivity using an Azure c300 Chemiluminescent Western Blot Imaging System.
(D) TTL3 abolishes the cytoplasmic retention of BZR1 by BIN2. Subcellular localization of BZR1-GFP alone, coexpressed with BIN2-HA, and with BIN2-HA and TTL3-HA in N. benthamiana leaves. Images of the GFP signal were obtained using laser scanning confocal microscopy. Images show a single equatorial plane in N. benthamiana leaves. Bar = 20 μm. The experiment was repeated three times with similar results.
(E) Immunoblot analysis of the BZR1-GFP proteins transiently expressed alone, coexpressed with BIN2-HA, and coexpressed with BIN2-HA and TTL3-HA in N. benthamiana leaves observed by confocal microscopy in (D). Proteins were analyzed by immunoblotting. Equal loading was confirmed by Coomassie blue staining (CBB) of input samples. BZR1-GFP was detected with anti-GFP antibody, while TTL3-HA and BIN2-HA were detected with anti-HA antibody. In the anti-GFP blot, the top band corresponds to phosphorylated BZR1 (pBZR1-GFP) and the bottom band to dephosphorylated BZR1 (BZR1-GFP).