Abstract
Implant-associated infection (IAI), a common condition marked by progressive inflammation and bone destruction, is mentally and financially devastating to those it affects, causing severe morbidity, prolonged hospital admissions, significant hospital costs and, in certain cases, mortality. Aspirin, a popular synthetic compound with a history of >100 years, is antipyretic, anti-inflammatory and analgesic. It is the most active component of non-steroidal anti-inflammatory drugs. However, the effects of aspirin on IAI remain unknown. In the present study, an IAI animal model was used, in which a stainless steel pin coated with Staphylococcus aureus was implanted through the left shaft of the tibia in mice. The animals were then randomized into five groups and subjected respectively to IAI, IAI + 15 mg aspirin treatment, IAI + 30 mg aspirin treatment, IAI + 60 mg aspirin treatment and IAI + 120 mg aspirin treatment groups. Aspirin was injected intraperitoneally twice daily for 11 days. Micro-CT and histological assays were performed to assess the effects of aspirin on IAI. It was found that aspirin reduced osteolysis and periosteal reaction, inhibited the activation of osteoclasts, promoted the activation of osteoblasts and facilitated healing of the infected fracture.
Keywords: aspirin, bacterial infection, osteolysis, Staphylococcus aureus
Introduction
Implant-associated infection (IAI), together with its progressive inflammation and bone destruction is catastrophic to patients physically, mentally and financially due to its significant morbidity and even mortality in certain cases (1). It is reported that ~60% of IAIs are caused by Staphylococcus aureus, which is difficult to treat as it has a variety of mechanisms to promote immune evasion, including the formation of a biofilm and persistence in necrotic bone (2).
Aspirin is a popular antipyretic, anti-inflammatory and analgesic drug and is the most active component of non-steroidal anti-inflammatory drugs (3). Recently, other roles of aspirin in disease treatment have been found. Cai et al (4) showed that aspirin enhanced the antibiotic-induced cell death of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). Zhou et al (5) also suggested that aspirin promoted the effectiveness of antibiotics against biofilm-associated infections. Sedlacek et al (6) showed that aspirin treatment significantly reduced the risk of Staphylococcus aureus in patients undergoing tunnel-catheter dialysis. Therefore, the present study hypothesized that aspirin may have beneficial effects on IAI. The aim of the present study was to examine the effects of aspirin on IAI.
To test the above hypothesis, an IAI animal model was used, in which a stainless steel pin coated with Staphylococcus aureus was implanted through the left shaft of the tibia. The findings demonstrated that aspirin may alleviate IAI.
Materials and methods
Staphylococcus aureus strain and pathogenic challenge
The strain of Staphylococcus aureus (ATCC43300) was purchased from Shanghai Luwei Microbial Science and Technology Co. Ltd. Pathogenic challenge was initiated using a contaminated 0.25-mm diameter pin that was generated as described by Li et al (1). The pins were autoclaved and stored in 75% ethanol. Following air-drying, the pins were incubated in 1.5 ml of an overnight broth culture of Staphylococcus aureus for 20 min. The pins were then air-dried for 5 min prior to trans-tibia implantation. The inoculating dose of bacteria was determined to be 1×106 CFU Staphylococcus aureus in PBS as Li et al described (1).
Animal surgery and treatment
Animal care and experimental procedures were approved by the Animal Care and Use Committee at the Southern Medical University, Guangzhou, China; NFYY-2017-31). A total of 40 6-week-old female C57BL/6J mice, purchased from the Experimental Animal Center of Southern Medical University, were used in the present study. The mice were housed in cages on a 12 h light/dark cycle (7:00 a.m.-7:00 p.m.), at 24°C and provided food and water ad libitum. Following acclimatization for 2 weeks, the 8-week-old mice with an average weight of 17±1 g were subjected to surgery as described by Li et al (1). The animals were anesthetized using 1% pentobarbitone sodium at a dose of 30 mg/kg via intraperitoneal injection. The vital signs (e.g., heart rate, respiratory rate and depth, color of mucous membranes and body temperature) and reflexes (e.g., toe pinch, tail pinch, eyelid/eyelash and palpebral) were observed to ensure the animals were deeply anesthetized (7). Their left tibiae were shaved, and 75% ethanol was used for sterilizing the skin. A pin was placed transcortically through the left tibia in a medial-to-lateral direction to initiate infection. The pin was bent at both ends for stability and cut adjacent to the skin on both ends, which allowed it to be covered by the skin and to eliminate additional environmental exposure. Once the mice had recovered from the anesthesia, they were returned to standard isolator cages and randomly divided into five groups to be subjected to the following: IAI, IAI + 15 mg aspirin treatment, IAI + 30 mg aspirin treatment, IAI + 60 mg aspirin treatment and IAI + 120 mg aspirin treatment. Aspirin was administrated via intraperitoneal injection twice daily for 11 days. The aspirin doses for the experimental mice were designed according to the equivalent ratios between the area of the human body surface and human doses of aspirin reported in the literature (8). For example, 60 mg aspirin treatment for a mouse is equivalent to the administration of 325 mg aspirin in a human on average.
Micro-CT analyses
Following sacrifice of the animals, the pins were removed, and the left tibiae were fixed in 4% p-formaldehyde for 24 h prior to scanning and histological evaluations of bone microstructures. The tibiae were scanned using a high-resolution micro-CT system (µCT 80, Scanco Medical, AG, Switzerland) with an isotropic voxel size of 12 µm (55 kV, 145 µA, integration time 300 ms, twice on average). The severity of osteolysis was determined by the size of the tibial hole determined by Image J (V1.8.0, official version) software (National Institutes of Health). Following 3D reconstruction of the CT images of the mice tibiae, the position of each reconstructed image was adjusted to reveal a circular hole as much as possible for determination of its area using Image J software. The area of the circular hole obtained minus the cross-sectional area of the pin implanted was calculated to determine the area of osteolysis. Statistical analysis of the osteolytic areas between the groups was conducted.
Histological and immunohistochemical (IHC) staining
Following micro-CT scanning, the tibiae were embedded in olefin following decalcification in 10% EDTA at room temperature for 4 weeks. All samples were sliced to a thickness of 6 µm and then disposed for histological and IHC analyses according to standard conditions. Gram-staining (Beijing Leagene Biotechnology Co., Ltd.) was used to evaluate bacterial content, hematoxylin and eosin staining (H&E) was performed to observe the histomorphology and inflammatory cell infiltration. H&E staining was performed using standard protocols and visualized with a light microscope (magnification, ×10 or ×20). Briefly, after dewaxing and hydration, the slices were stained with hematoxylin in refined aqueous solution for 2 min at room temperature, differentiated in hydrochloric acid ethanol for ≤10 sec, rinsed in running water for 15 min, and then placed in distilled water for 1 min. The sections were then dehydrated in 70 and 90% alcohol for 10 min, respectively, and stained with alcohol eosin solution for 2-3 min at room temperature.
Additionally, following blocking with 10% goat serum (cat. no. PK-4001; Vector Laboratories, Inc.) for 1 h at room temperature, IHC staining of osterix (OST; Abcam Cambridge, UK) was performed to evaluate the activity of osteoblasts around the IAI site. According to the standard protocol, primary antibodies against OST (1:400; cat. no. ab22552; Abcam) were applied for incubation overnight at 4°C, followed by incubation with a biotinylated goat anti-rabbit secondary antibody (1:200; cat. no. PK-4001; Vector Laboratories, Inc.) and DAB staining (Dako; Agilent Technologies, Inc.) at room temperature for 3 min. Subsequently, the slides were immersed with hematoxylin (cat. no. H3136; Sigma-Aldrich; Merck KGaA) at room temperature for 5 min. The slides were then visualized with a light microscope (magnification, ×10 or ×20). By calculating the brown-stained cells among the total cells in three randomly selected fields, the relative numbers of OST positive cells were quantified. Tartrate-resistant acid phosphatase (TRAP) (Sigma-Aldrich; Merck KGaA) staining was performed at room temperature for 1 h to evaluate the presence of osteoclasts around the IAI site, according to the standard protocol. TRAP positive cells with three or more nuclei were quantified under a light microscope (magnification, ×20). Furthermore, Goldner's staining (Beijing Solarbio Science & Technology Co., Ltd.) was used to assess the activity of the osteoblasts and osteoclasts according to the standard protocol.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Continuous data are presented as the mean + standard deviation. One-way analysis of variance followed by Dunnett's test was used for continuous variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Aspirin reduces osteolysis and periosteal reaction
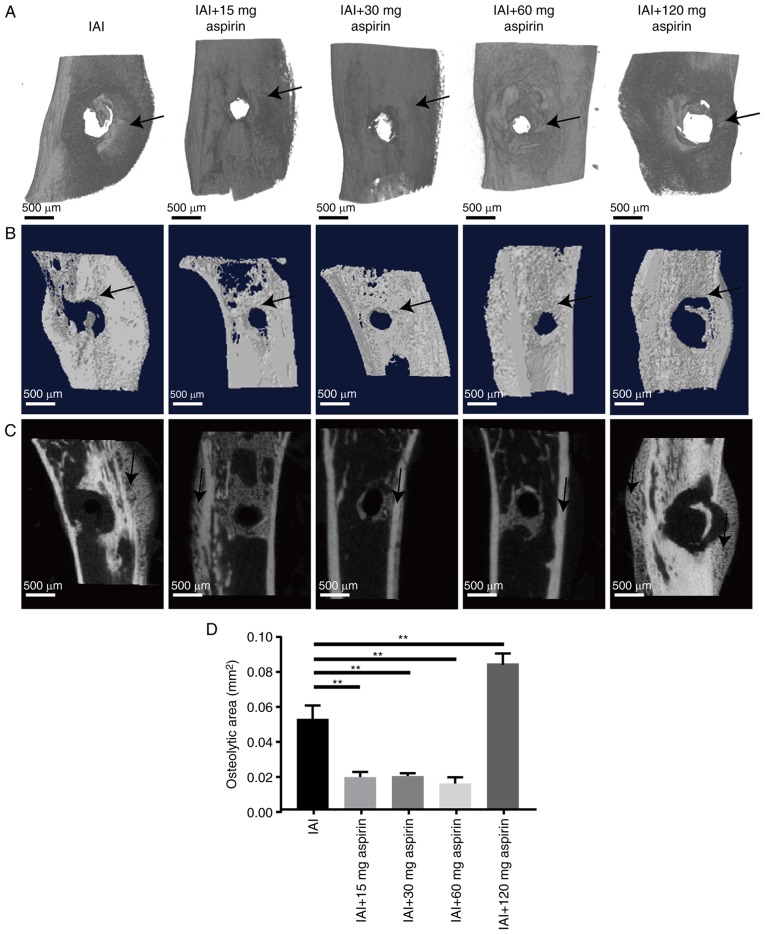
The micro-CT analysis demonstrated that osteolysis and periosteal reaction were reduced in the groups treated with aspirin at doses of 15, 30 and 60 mg in a dose-dependent manner. However, 120 mg aspirin had an adverse effect on osteolysis and periosteal reaction (Fig. 1). As shown in Fig. 1A and B, bone destruction and osteolysis in the IAI group were more marked than those in the three groups treated with aspirin at doses of 15, 30 and 60 mg, as the hole in the IAI group was clearly larger in area than that in either of the three groups. The area of the hole decreased from the group treated with 15 mg aspirin to that treated with 60 mg aspirin. It is notable that the differences in the osteolytic area between the 15, 30 and 60 mg aspirin groups were not statistically significant. However, bone destruction and osteolysis in the 120 mg aspirin group were more marked than those in the IAI group as the hole in the former group had a significantly larger area than that in the latter (P<0.05). As shown in Fig. 1C, the periosteal reaction in the IAI group was significantly greater than that in the groups treated with 15, 30 and 60 mg aspirin, but did not differ from that in the group treated with 120 mg aspirin. The gross observation (Fig. 1C) showed that the periosteal reaction in the IAI group was more intense compared with the groups treated with 15, 30 and 60 mg aspirin; however, no obvious difference was noted compared with the group treated with 120 mg aspirin. Taken together, these data indicate that well-dosed aspirin may effectively reduce the osteolysis and periosteal reaction caused by bacterial infection, but excessive aspirin may aggravate the osteolysis and periosteal reaction. The graph in Fig. 1D shows the statistical analysis of osteolytic areas between the groups. Aspirin at doses of 15, 30 and 60 mg led to a significantly smaller osteolytic area than that in the IAI and 120 mg aspirin groups. However, it is notable that differences in the osteolytic areas between the 15, 30 and 60 mg aspirin groups were not statistically significant.
Figure 1.
Micro-CT images of left tibias. (A) Three-dimensional reconstruction showing bone destruction and osteolysis (hole). (B) Transverse sections of the three-dimensional reconstruction showing bone destruction and osteolysis (hole). The black arrows indicate the hole measured. (C) 2D images of a sagittal sections. The black arrows indicate the site of periosteal reaction. (D) Statistical analysis of bone destruction and osteolysis. Data are presented as the mean ± standard deviation. **P<0.01. IAI, implant-associated infection.
Aspirin decreases infection and alleviates inflammatory response
A representative graph is presented in Fig. 2A showing the infection 11 days after IAI modeling in each group. The bacterial infection in the IAI group resulted in skin ulceration and substantial pus around the pin. In the 15, 30 and 60 mg aspirin groups, there appeared to be only slight redness and swelling. No obvious fluctuation of pus was found in the 15, 30 and 60 mg aspirin groups. The least redness and swelling was observed in the 60 mg aspirin group. However, in the 120 mg aspirin group, no ulceration was observed but there was obvious redness and fluctuation of pus, suggesting pus accumulation in the local area. The results of the H&E staining showed that the inflammatory cell infiltration in the IAI group was significantly greater than that in the aspirin treatment groups (Fig. 2B). The 60 mg aspirin group had the least inflammatory cell infiltration, suggesting it had the optimal effect on alleviating the local inflammatory response caused by the bacteria. Gram-staining showed no bacterial content in any of the groups, which may have been due to the fact that the duration of modeling was not sufficient for bacterial colonies to have formed on the surface of the bone (Fig. 2C). The results of the statistical analysis of infiltrating inflammatory cells in tissue sections in each group are shown in Fig. 2D. Aspirin doses of 15, 30 and 60 mg led to significant decreases in the number of infiltrating inflammatory cells in a dose-dependent manner. However, no obvious decrease in the number of infiltrating inflammatory cells was observed in the 120 mg aspirin group.
Figure 2.
Inflammatory cell infiltration. (A) General view of the left tibia 11 days after pathogenic challenge. The black arrows indicate the site of pathogenic challenge. (B) H&E staining. The black arrows indicate inflammatory cells. (C) Gram staining. (D) Statistical analysis of inflammatory cell infiltration in each group. Data are presented as the mean ± standard deviation. **P<0.01. IAI, implant-associated infection; H&E, hematoxylin and eosin.
Aspirin augments bone formation
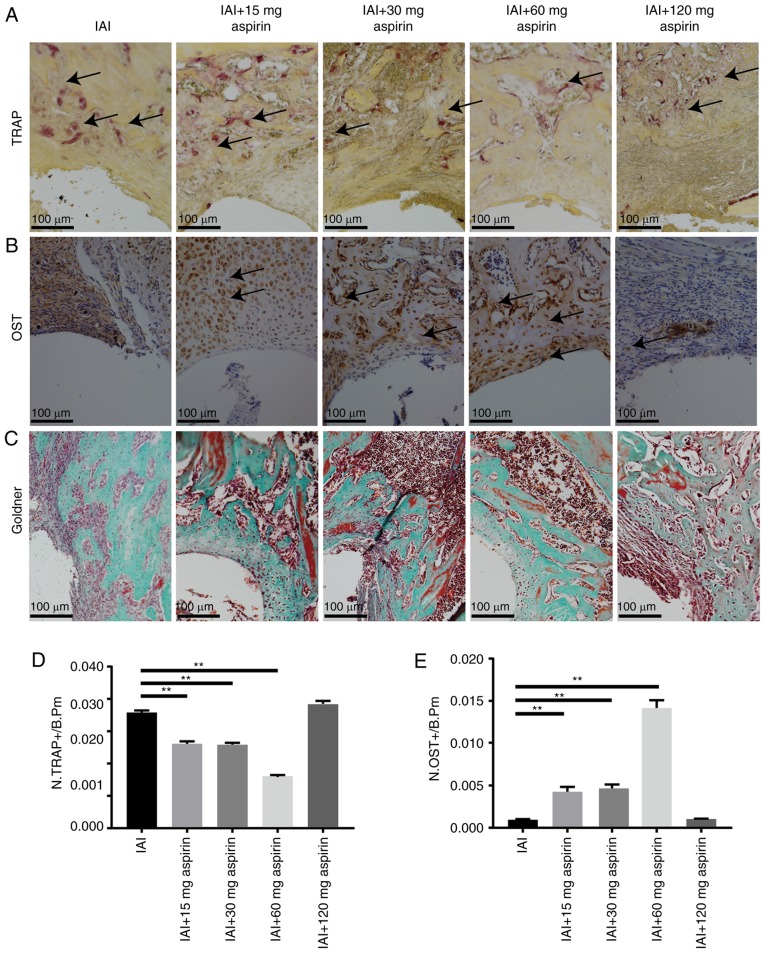
The results in Fig. 3A and B show significantly lower expression of TRAP and markedly higher expression of OST in the 15, 30 and 60 mg aspirin groups compared with expression in the IAI group (P<0.05), but there were no significant differences in the expression of TRAP or OST between the 120 mg aspirin group and the IAI group. The formation of bone increased from the group treated with 15 mg aspirin to that treated with 60 mg aspirin. Goldner's staining demonstrated the same results with TRAP and OST. The 15, 30 and 60 mg aspirin groups had increased the formation of osteoid and mineralized bone, whereas the 120 mg aspirin group had no effect on the formation of osteoid and mineralized bone (Fig. 3C). The results of the TRAP staining, IHC staining of OST and Goldner's trichrome staining showed that aspirin at an appropriate concentration promoted osteogenesis following IAI and reduced the osteolysis caused by infection. The results in Fig. 3D show the statistical analysis of TRAP-positive cells between groups. Aspirin doses of 15, 30 and 60 mg led to significantly decreased expression of TRAP-positive cells in a dose-dependent manner. The statistical analysis of OST-positive cells between groups is shown in Fig. 3E. Aspirin doses of 15, 30 and 60 mg led to significantly increased expression of OST-positive cells in a dose-dependent manner.
Figure 3.
Staining of osteoclasts and osteoblasts for evaluation of bone formation. (A) TRAP staining. The black arrows indicate osteoclast-positive cells (TRAP). (B) Immumohistochemical staining of osteoblast-positive cells (OST). The black arrows indicate osteoblast-positive cells. (C) Goldner's staining. Statistical analysis of the (D) TRAP-positive cells (N.TRAP+) and (E) OST-positive cells (N.OST+) on the bone surface. Data were measured as cells per millimeter of perimeter in sections and are presented as the mean + standard deviation. **P<0.01. B.pm, cells per millimeter of perimeter in sections.
Discussion
Taken together, the experimental findings in the present study demonstrated that aspirin alleviated IAI in a dose-dependent manner below a dose of 60 mg but had an adverse effect on IAI at a high dose. Treatment with moderate doses of aspirin resulted in decreased periosteal reactive bone, osteolysis and osteoclast activation but increased osteoblast activation in IAI mice, whereas a high dose of aspirin aggravated rather than alleviated IAI.
The benefit of aspirin for IAI may be attributed to a variety of factors. Firstly, PGE2 may increase biofilm formation in Staphylococcus aureus and MRSA (4). It is known that aspirin can inhibit the production of prostaglandin, which can be a significant virulence factor in biofilm-associated infections (9). Furthermore, the impact of aspirin on platelets may also be of benefit as platelets are a host factor in the circulation to consolidate biofilm formation and protect bacteria against antibiotics. Additionally, as aspirin is an efficient inhibitor of quorum sensing, virulence and toxins, it may also have a beneficial effect on IAI (10). Finally, the effect of aspirin on biofilm is important. Cyclooxygenase inhibitors including aspirin can reduce biofilm formation (11). A recent study demonstrated that salicylic acid (SAL) reduced bacterial adhesion, affected biofilm structural development, reduced viable biomass, extracellular proteins and polysaccharides, and promoted biofilm detachment (12). However, it was observed that the presence of SAL, the active component of aspirin, exhibited moderate iron-chelating capacity, which markedly promoted Staphylococcus aureus biofilm production (13). These different observations may have been due to differences in the experiment design, model use and aspirin concentrations.
The present study demonstrated that aspirin reduced periosteal reactive bone, osteolysis and osteoclast activation but promoted osteoblast activation. This can be explained by the capabilities of aspirin, including promoting the recruitment, migration and osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs), enhancing the effect of osteoblasts and inhibiting the effect of osteoclasts (14,15). Of note, treatment with 120 mg aspirin resulted in reduced infection but significantly increased bone destruction. The possible reason for this may be that aspirin at an excessive concentration may have led to the immoderate inhibition of certain inflammatory factors that may be helpful for osteogenesis or that an intemperate concentration of aspirin may have had toxic effects on BMSCs and osteoblasts. Similarly, Lack et al observed that a dose of aspirin above a threshold approximating a single human dose of 325 mg was associated with delayed bone healing (16). Furthermore, Dapunt et al (17) found that osteoblasts expressed the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus. Taken together, the results of the present study demonstrated that bacterial challenge to osteoblasts during IAI induced the cells to produce inflammatory molecules that directly appropriated host responses or contributed to progressive inflammatory damage, resulting in bone destruction which was weakened by aspirin.
The optimal effect of aspirin on the alleviation of bone infection was observed at a dose of 60 mg. A significantly lower expression level of TRAP and a markedly higher expression level of OST were observed in the 15, 30 and 60 mg aspirin groups compared with those in the IAI group in a dose-dependent manner, indicating that an aspirin dose of 60 mg may result in the least bone destruction and the most bone formation. The observation that the hole (osteolysis) in the IAI group was larger in area than that in the three groups treated by aspirin at doses of 15, 30 and 60 mg indicated that the bone destruction and osteolysis in the IAI group were more marked than those in the three groups. The area of the hole (osteolysis) decreased from the group treated with 15 mg aspirin to that treated with 60 mg aspirin, however, the differences in osteolytic areas among 15, 30 and 60 mg aspirin groups were not statistically significant according to the micro-CT images. That the differences in the osteolytic area among the three concentrations of aspirin were not significant demonstrated the similar effects of the three treatments. It may be that the similar effects resulted from a similar offset between bone formation and bone destruction to different extents. In addition, changes in bone structure may have appeared at a slower rate than the histological changes in cells. The timescale of only 11 days for observing changes in bone structure sampled from the animal model was not long enough; if IAI modeling and aspirin treatment had been prolonged, the results may have been significantly different from those obtained in the present study. These possibilities require further experiments for clarification.
It was found that the 15, 30 and 60 mg aspirin groups exhibited significantly lower expression of TRAP and markedly higher expression of OST than the IAI group. Furthermore, Goldner's staining demonstrated the same results as the TRAP and OST staining. The 15, 30 and 60 mg aspirin groups exhibited apparently increased formation of osteoid and mineralized bone. TRAP is synthesized by osteoclasts, thus reflecting the status of osteoclasts. The expression of TRAP-positive cells is increased when bone resorption increases. OST is a transcription factor with a zinc finger structure that is important for bone formation. The expression of OST-positive cells is increased when bone formation increases. Goldner's trichrome staining is usually used for bone tissue staining, with green indicating mineralized bone, orange and red osteoid, purple cartilage, and blue or gray nuclei.
Aspirin is now widely used in the human population to prevent cardiovascular and cerebrovascular diseases. Recent studies indicate that patients undergoing spinal surgery with continued aspirin administration do not have an increased risk of bleeding and that no increase has been observed in the surgery duration or postoperative blood transfusion (18,19). Studies have demonstrated that aspirin is as effective as rivaroxaban and warfarin for prophylaxis of venous thromboembolism and can also reduce postoperative infection rates (20-22). In the present study, it was demonstrated that aspirin alleviated orthopedic IAI and promoted healing of the infected fracture. As aspirin is more cost-effective than other anticoagulant drugs and has a beneficial effect on IAI, it may be used in orthopedic surgery as a potent candidate drug. Further investigations are required to clarify the clinical benefits of aspirin.
The present study had several limitations. It was not possible to fully imitate the clinical implant-related infection in the animal models used. In addition, aspirin administration was not conducted orally as in a routine clinical manner. An important limitation is that only imaging and histological evaluations were used to observe the effect of aspirin on IAI, whereas no investigations were performed to examine the in-depth mechanisms. There was also no investigation of the type of infection or the type of cells recruited to the affected area. Although it is known that the early stage of infection is mainly associated with the infiltration of neutrophils and that the middle and late stages of infection are associated with the infiltration of macrophages and lymphocytes (1), it may have been informative to identify the types of inflammatory cells present. Furthermore, IHC staining was used to detect the osteoblast-associated protein expression and TRAP was used to detect the osteoclast-associated protein expression, as the literature indicates that IHC and TRAP are sufficient to demonstrate osteoclast/osteoblast dynamics (14,15); however, immunofluorescence staining may have assisted in consolidating the osteoclast/osteoblast dynamics. There is no doubt that the in-depth mechanisms of how aspirin may act on IAI and the related osteoclast/osteoblast dynamics are the chief concerns of our future investigations.
In a preliminary observation of the effects of aspirin on IAI, the present study found that aspirin may alleviate IAI and reduce periosteal reactive bone, osteolysis and osteoclast activation but promote osteoblast activation, facilitating healing of the infected fracture. These findings and additional details of the effects of aspirin on IAI require further investigation by further large animal experiments and clinical trials. The mechanisms underlying how and to what extent aspirin may affect IAI also require further elucidation.
Acknowledgments
The authors would like to thank Professor Ping Allen Liang (Chinese Journal of Orthopaedic Trauma, Guangzhou, China) for his assistance with the revision of the manuscript and his careful proofreading.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81572165) and Guangdong Provincial Science and Technology Department Plan Projects (grant no. 2013B090600140).
Availability of data and materials
The corresponding author can be contacted for data requests.
Authors' contributions
All authors were involved in the conception and design of the study, or in the acquisition analysis and interpretation of data, and in critical revision for important intellectual content. Experiments were designed by YJ and BY. The experiments were performed by YJ, SW, HQ, HW, MR and JL. Data were analyzed by YJ, SW and BY. The paper was written by YJ and BY. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All aspects of the research were conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee of Nanfang Hospital, Southern Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li D, Gromov K, Søballe K, Puzas JE, O'Keefe RJ, Awad H, Drissi H, Schwarz EM. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008;26:96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farnsworth CW, Schott EM, Benvie AM, Zukoski J, Kates SL, Schwarz EM, Gill SR, Zuscik MJ, Mooney RA. Obesity/type 2 diabetes increases inflammation, periosteal reactive bone formation, and osteolysis during Staphylococcus aureus implant-associated bone infection. J Orthop Res. 2018;36:1614–1623. doi: 10.1002/jor.23831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Xu CG, Zhou YB, Hao YH, Ren MQ, Wang YZ, Chen XT, Muhammad JQ, Wang I, Liu SD, et al. Comparative proteomic analysis provides insight into the key proteins as possible targets involved in aspirin inhibiting biofilm formation of staphylococcus xylosus. Front Pharmacol. 2017;8:543. doi: 10.3389/fphar.2017.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai JY, Hou YN, Li J, Ma K, Yao GD, Liu WW, Hayashi T, Itoh K, Tashiro SI, Onodera S, Ikejima T. Prostaglandin E2 attenuates synergistic bactericidal effects between COX inhibitors and antibiotics on Staphylococcus aureus. Prostaglandins Leukot Essent Fatty Acids. 2018;133:16–22. doi: 10.1016/j.plefa.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J. In vitro interactions between aspirin and ampho-tericin B against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother. 2012;56:3250–3260. doi: 10.1128/AAC.06082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sedlacek M, Gemery JM, Cheung AL, Bayer AS, Remillard BD. Aspirin treatment is associated with a significantly decreased risk of Staphylococcus aureus bacteremia in hemodialysis patients with tunneled catheters. Am J Kidney Dis. 2007;49:401–408. doi: 10.1053/j.ajkd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann K, Flecknell P. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX. 2019;36:65–80. doi: 10.14573/altex.1804011. [DOI] [PubMed] [Google Scholar]
- 8.Bethel MA, Harrison P, Sourij H, Sun Y, Tucker L, Kennedy I, White S, Hill L, Oulhaj A, Coleman RL, Holman RR. Randomized controlled trial comparing impact on platelet reactivity of twice-daily with once-daily aspirin in people with Type 2 diabetes. Diabet Med. 2016;33:224–230. doi: 10.1111/dme.12828. [DOI] [PubMed] [Google Scholar]
- 9.Alem MA, Douglas LJ. Prostaglandin production during growth of Candida albicans biofilms. J Med Microbiol. 2005;54:1001–1005. doi: 10.1099/jmm.0.46172-0. [DOI] [PubMed] [Google Scholar]
- 10.El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog. 2014;74:25–32. doi: 10.1016/j.micpath.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Abdelmegeed E, Shaaban MI. Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of flucon-azole resistant Candida albicans. J Microbiol. 2013;51:598–604. doi: 10.1007/s12275-013-3052-6. [DOI] [PubMed] [Google Scholar]
- 12.Cattò C, Grazioso G, Dell'Orto S, Gelain A, Villa S, Marzano V, Vitali A, Villa F, Cappitelli F, Forlani F. The response of Escherichia coli biofilm to salicylic acid. Biofouling. 2017;33:235–251. doi: 10.1080/08927014.2017.1286649. [DOI] [PubMed] [Google Scholar]
- 13.Dotto C, Lombarte Serrat A, Cattelan N, Barbagelata MS, Yantorno OM, Sordelli DO, Ehling-Schulz M, Grunert T, Buzzola FR. The active component of aspirin, salicylic acid, promotes staphylococcus aureus biofilm formation in a PIA-dependent manner. Front Microbiol. 2017;8:4. doi: 10.3389/fmicb.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, Liu Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210. doi: 10.1186/s13287-015-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lack WD, Fredericks D, Petersen E, Donovan M, George M, Nepola J, Smucker J, Femino JE. Effect of aspirin on bone healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2013;95:488–496. doi: 10.2106/JBJS.L.00462. [DOI] [PubMed] [Google Scholar]
- 17.Dapunt U, Maurer S, Giese T, Gaida MM, Hänsch GM. The macrophage inflammatory proteins MIP1α (CCL3) and MIP2α (CXCL2) in implant-associated osteomyelitis: Linking inflammation to bone degradation. Mediators Inflamm. 2014;2014:728619. doi: 10.1155/2014/728619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goes R, Muskens IS, Smith TR, Mekary RA, Broekman MLD, Moojen WA. Risk of aspirin continuation in spinal surgery: A systematic review and meta-analysis. Spine J. 2017;17:1939–1946. doi: 10.1016/j.spinee.2017.08.238. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Wang G, Liu X, Li Y, Sun J. Safety of continuing aspirin therapy during spinal surgery: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8603. doi: 10.1097/MD.0000000000008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang RC, Parvizi J, Hozack WJ, Chen AF, Austin MS. Aspirin is as effective as and safer than warfarin for patients at higher risk of venous thromboembolism undergoing total joint arthroplasty. J Arthroplasty. 2016;31:83–86. doi: 10.1016/j.arth.2016.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Huang R, Buckley PS, Scott B, Parvizi J, Purtill JJ. Administration of aspirin as a prophylaxis agent against venous thromboembolism results in lower incidence of periprosthetic joint infection. J Arthroplasty. 2015;30:39–41. doi: 10.1016/j.arth.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Colleoni JL, Ribeiro FN, Mos PAC, Reis JP, Oliveira HR, Miura BK. Venous thromboembolism prophylaxis after total knee arthroplasty (TKA): Aspirin vs. rivaroxaban. Rev Bras Ortop. 2017;53:22–27. doi: 10.1016/j.rbo.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author can be contacted for data requests.