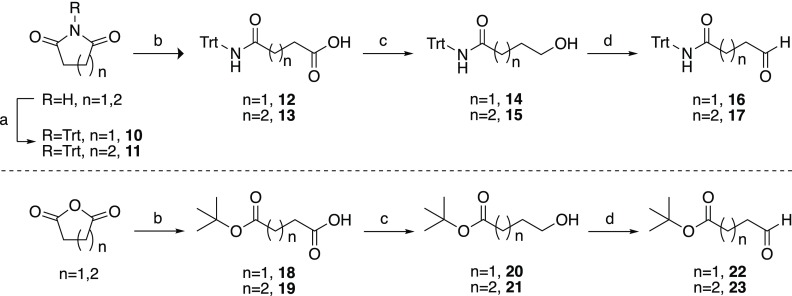
Scheme 2. Synthetic Route for Aldehydes 16, 17, 22, and 23.
Reagents and conditions: (a) TrtCl, CH3CN, K2CO3, rt, 48 h (20–28%); (b) KOH, EtOH, reflux, overnight (37–93%) (c) NaBH4, BF3·Et2O, THF, 0 °C to rt, 2 h (64–81%); (d) PDC, CH2Cl2, rt, 2 h (65–78%); (e) tert-butanol, 4-dimethylaminopyridine (DMAP), N-hydroxysuccinimide, Et3N, toluene, overnight (25–93%).