Abstract
Atherosclerosis is a major pathogenic factor in patients with cardiovascular diseases, and endothelial dysfunction (ED) plays a primary role in its occurrence and development. Simvastatin is a lipid-lowering drug, which is commonly used to prevent or treat risk factors of cardiovascular diseases with a significant anti-atherogenic effect. However, its impact on endothelial cells under conditions of oxidative stress and broader mechanisms of action remain unclear. The present study evaluated the effect of simvastatin on human umbilical vein endothelial cells (HUVECs) under oxidative stress with H2O2, and the associated mechanisms. At a high dose (1 µM), simvastatin exacerbated H2O2-induced endothelial cell dysfunction. Moreover, inhibition of the Wnt/β-catenin pathway by salinomycin significantly suppressed the simvastatin-associated HUVEC dysfunction. Western blot analysis further demonstrated that simvastatin promoted the phosphorylation of low-density lipoprotein receptor-related protein 6 (LRP6) and activated the Wnt/β-catenin pathway. Simvastatin also activated endoplasmic reticulum (ER) stress, which was reversed by salinomycin treatment. Based on these results, it was hypothesized that simvastatin may promote ER stress by facilitating LRP6 phosphorylation and the subsequent activation of the Wnt/β-catenin pathway, thereby enhancing H2O2-induced ED. Therefore, high-dose simvastatin treatment could have potential toxic side effects, indicating the need for close clinical management, monitoring and patient selection.
Keywords: simvastatin, oxidative stress, ER stress, Wnt/β-catenin pathway, ED, HUVECs
Introduction
Atherosclerosis (AS) is a pathological condition characterized by artery narrowing, and is associated with high morbidity and mortality rates worldwide. Although the detailed pathogenic mechanisms remain to be elucidated, endothelial dysfunction (ED) is the initiating event of AS (1,2), which can alter the homeostasis of the cardiovascular system, with accompanying changes in cell morphology and function. The canonical Wnt/β-catenin pathway plays a crucial role in cell proliferation, adhesion and other physiological processes (3), and has been implicated in various conditions, such as inflammatory disease, fibrotic disease and multiple cancer types (4,5). Previous studies have suggested that this pathway is also related to the development of AS (6,7). Indeed, Wnt/β-catenin participates in the inflammatory response, which may cause ED (8). Moreover, previous findings have demonstrated that sphingomyelin synthase 2 overexpression may activate the Wnt/β-catenin pathway and potentially lead to ED (9,10).
AS has also been associated with endoplasmic reticulum (ER) stress, which is related to numerous cellular biological functions (11). ER stress is induced by unfolded proteins when homeostasis of the ER is disrupted by adverse conditions, such as hyperlipidaemia and oxidative stress (12,13). Animal experiments have shown that the expression levels of the ER stress-associated proteins 78 kDa glucose-regulated protein (GRP78), phosphorylated (phospho)-PRKR-like endoplasmic reticulum kinase, phosphoserine/threonine-protein kinase/endoribonuclease IRE1, cyclic AMP-dependent transcription factor ATF-6α (ATF6) and C/EBP-homologous protein (CHOP) were increased in apolipoprotein E-knockout mice. In addition, many atherogenic risk factors can activate ER stress in the initial stages of AS, further aggravating ED and AS (14,15). Moreover, Hong et al (16) proposed ER stress as an important contributing factor to ED during AS development and progression. Thus, ER stress might contribute to AS by promoting ED.
Simvastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, and its ability to reduce cholesterol and blood lipids is clinically exploited to lower the risk of cardiovascular events (17). However, its effects on endothelial cells under oxidative stress conditions and the possible underlying mechanisms are still unclear. In addition, statins can decrease the rate of neural apoptosis and promote neural recovery by activating the Wnt/β-catenin pathway (18). Notably, in functional recovery from traumatic brain injury, simvastatin reinforces neurogenesis via the regulation of isoprenoid synthesis and the activation of Wnt signalling (19). Most of these previous results support the beneficial effects of simvastatin. However, the present study revealed that high doses of simvastatin (≥1 µM) may even induce ED. Simvastatin regulates the Wnt/β-catenin pathway in nerve cells; however, its effect on the Wnt/β-catenin pathway in relation to ER stress contributing to ED is not clear. To examine the potential impact of simvastatin on ED, in the present study, human umbilical vascular endothelial cells (HUVECs) were subjected to H2O2-induced oxidative stress in the presence of simvastatin and/or the Wnt/β-catenin inhibitor salinomycin, and the level of ED, as well as the expression of proteins related to the Wnt/β-catenin pathway and ER stress, were evaluated. These findings may contribute to gaining an improved understanding of the molecular and cellular mechanisms regulating AS, and offer an insight into the risks and mechanisms of action of the clinical use of statins, while highlighting new targets for treatment and prevention.
Materials and methods
Cell culture
HUVECs (Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) were cultivated in Dulbecco's modified Eagle's medium containing penicillin and streptomycin (100 U/ml and 0.1 mg/ml, respectively; Beijing Solarbio Science & Technology Co., Ltd.) plus 10% certified foetal bovine serum (FBS; Biological Industries). In addition, THP-1 cells (Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) were used in a co-culture experiment to assess the effect of the treatments on the adhesion ability of HUVECs, which may reflect the degree of ED. THP-1 cells were cultured in RPMI-1640 (Beijing Solarbio Science & Technology Co., Ltd.) plus 10% FBS. All cells were cultured at 37°C in a 5% CO2 atmosphere.
Treatment of HUVECs with simvastatin and salinomycin
The cells were cultured as described above, and then treated with 1 µM simvastatin (Dalian Meilun Biology Technology Co., Ltd.) and/or 10 µM salinomycin (MedChemExpress) for 24 h. Next, the cells were treated with H2O2 (500 µM) for another 24 h to induce oxidative stress. Therefore, the following four treatment groups were created: Control (H2O2); Sim (Simvastatin+H2O2); Sal (Salinomycin+H2O2); and Sim+Sal (Simvastatin+Salinomycin+H2O2). Supernatants and cells were collected for further analysis. Each treatment group consisted of six replicate wells, and each experiment was performed in triplicate. All cells were cultured at 37°C in a 5% CO2 atmosphere.
Cell viability assay
HUVECs were seeded in 96-well plates at 80% confluence and were exposed to different concentrations of simvastatin (0, 1, 2 and 4 µM) for 24 h. The cells were also treated with simvastatin (1 µM) and/or salinomycin (10 µM) for 24 h, and then incubated with H2O2 (500 µM) for another 24 h. To assess cell viability, 20 µl MTT (5 mg/ml; cat. no. M8180; Beijing Solarbio Bioscience & Technology Co., Ltd.) was added to each well and the cells were further incubated for 4 h. The medium was removed, 150 µl dimethyl sulfoxide was added to each well, and the plates were agitated for 15 min. The absorbance was measured at 490 nm. All cells were cultured at 37°C in a 5% CO2 atmosphere.
Acridine orange (AO) and ethidium bromide (EB) staining
HUVECs were added into 24-well plates, grown to 80% confluence, and the aforementioned treatments were applied. The cells were stained with 5 µl AO and 5 µl EB (Solarbio Life Science) for 5 min at room temperature in the dark. The dual stain was then removed, and the cells washed with PBS three times. Fluorescence was observed under a fluorescence microscope (magnification, ×20; Olympus IX71; Olympus Corporation).
Measurement of the lactate dehydrogenase (LDH) level in the medium, intracellular superoxide dismutase (SOD), nitric oxide synthase (NOS) activity and malondialdehyde (MDA) content
HUVECs in 6-well plates at a confluence of 80% were treated in the aforementioned manner, and the medium was collected for the measurement of LDH release levels (cat. no. A020-1; Nanjing Jiancheng Bioengineering Institute) based on the absorbance measured at 450 nm, indicating the extent of cell injury. The cells were harvested and centrifuged (1,520 × g; 5 min; room temperature). Homogenates were obtained after cell breakage by sonication, set at an overall duration of 5 min and performed at a frequency of 20 kHZ every 30 sec for 5 sec at 4°C. SOD (cat. no. A001-1; Nanjing Jiancheng Bioengineering Institute) and nitric oxide synthase NOS (cat. no. A014-1; Nanjing Jiancheng Bioengineering Institute) activities, as well as the MDA (cat. no. A003-1; Nanjing Jiancheng Bioengineering Institute) intracellular content were determined using the respective kits, according to the manufacturer's protocol, based on the absorbance measured at 560 nm.
Cell adhesion assay
After treatment with simvastatin and/or salinomycin, followed by exposure to H2O2, THP-1 monocytic cells were added to the medium of HUVECs at a density of 5×103/well, and culturing was continued for 30 min. Next, the cells were washed twice with PBS so that only the THP-1 cells adhering to the HUVECs remained in the wells. Finally, THP-1 cells on HUVECs were counted under a phase-contrast inverted microscope (magnification, ×20; Olympus IX71; Olympus Corporation) to assess the cell adhesion ability. The average number of THP-1 cells was obtained from at least three replicate experiments.
Western blot analysis
Total proteins were extracted from the cells using radioimmunoprecipitation assay buffer (Beijing Solarbio Science & Technology Co., Ltd.), quantified using a bicinchoninic acid kit, and ~60 µg protein/lane was separated via SDS-PAGE on an 8-12% gel. Polyvinylidene fluoride membranes (Immobilon-P; EMD Millipore) were used to transfer the separated proteins. Blocking was performed with 5% skimmed milk or 10% bovine serum albumin (Beijing Solarbio Science & Technology Co., Ltd.) dissolved in TBS with 0.05% Tween-20 (TBST) for 1 h at room temperature. Next, the following primary antibodies were added to TBST, and incubated for at least 8 h at 4°C: Antibodies against the apoptosis-associated proteins Bcl-2 (cat. no. 60178-1-Ig; 1:1,000; ProteinTech Group, Inc.) and Bax (cat. no. 505992-2-Ig; 1:2,000; ProteinTech Group, Inc.); the adhesion molecules intercellular cell adhesion molecule 1 (ICAM-1; cat. no. 10831-1-AP; 1:1,000; ProteinTech Group, Inc.), vascular cell adhesion protein 1 (VCAM-1; cat. no. WL02474; 1:500; Wanleibio Co., Ltd.) and monocyte chemoattractant protein 1 (MCP-1; cat. no. WL01755; 1:1,000; Wanleibio Co., Ltd.); the Wnt/β-catenin pathway-associated proteins β-catenin (cat. no. 51067-2-AP; 1:2,000; ProteinTech Group, Inc.), phospho-β-catenin (cat. no. DF2989; 1:1,000; ProteinTech Group, Inc.), low-density lipoprotein receptor-related protein 6 (LRP6; cat. no. A13325; 1:1,000; ABclonal Biotech Co., Ltd.) and phospho-LRP6 (cat. no. abs140173; 1:1,000; Absin Bioscience, Inc.); and the ER stress-associated proteins GRP78 (cat. no. 66574-1-Ig; 1:5,000; ProteinTech Group, Inc.), CHOP (cat. no. WL00880; 1:800; Wanleibio Co., Ltd.) and ATF6 (cat. no. Wl02407; 1:800; Wanleibio Co., Ltd.). Protein content was normalized using GAPDH (cat. no. HRP-60004; 1:8,000; ProteinTech Group, Inc.) as the internal control. The membrane was subsequently washed three times and incubated with horseradish peroxidase-conjugated anti-rabbit (cat. no. BA1054; 1:8,000; Boster Biological Technology) and anti-mouse (cat. no. SA0000I-I; 1:8,000; ProteinTech Group, Inc.) secondary antibodies in TBST for 1 h at room temperature. Following three additional washes, bands were detected by enhanced chemiluminescence (cat. no. WLA006c; Wanleibio Co., Ltd.) and autoradiography (Chemiluminescence Imaging system; version 5.1; Bio-Rad Laboratories, Inc.). All experiments were performed in triplicate. The results were assessed using Image Lab software (version 5.1; Bio-Rad Laboratories, Inc.).
Statistical analysis
The data were analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc.). Significant differences between groups were determined using one-way ANOVA followed by Tukey's post-hoc analysis. Data are presented as the mean ± SD from at least three independent experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of simvastatin on HUVECs and its possible mechanisms
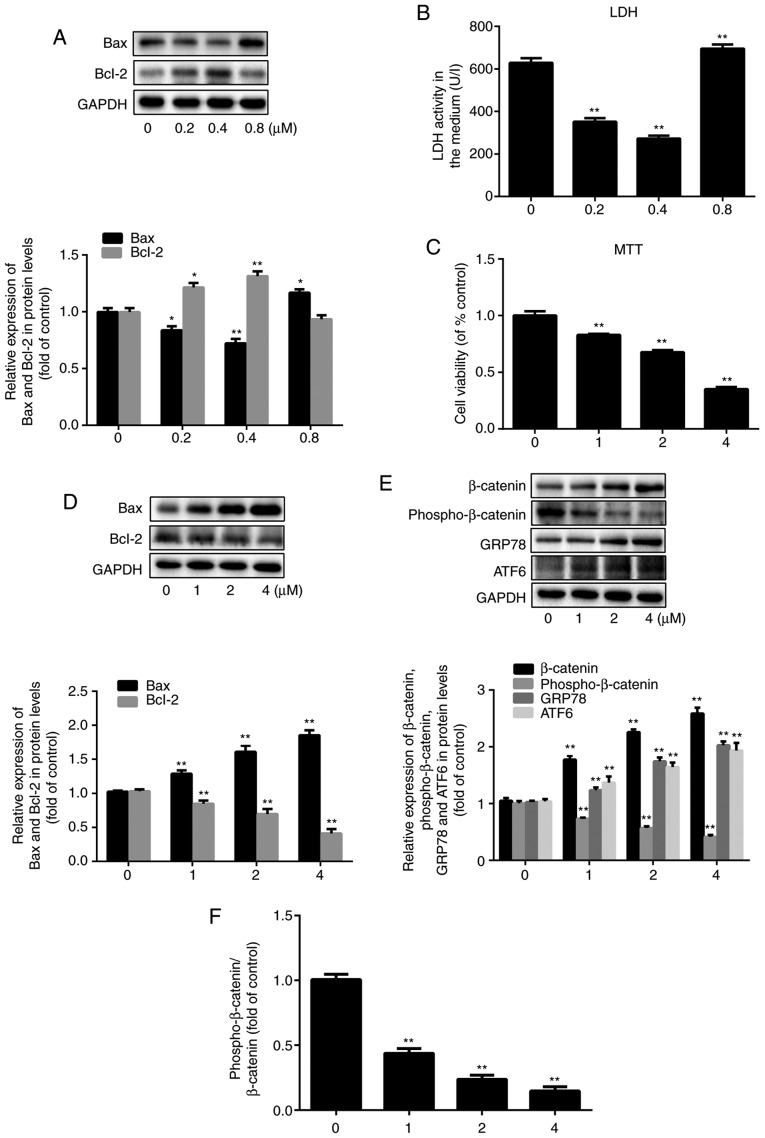
To study the impact of simvastatin on the viability of HUVECs pre-exposed to oxidative stress, two different concentration series were tested. At the lower concentrations of 0.2 µM and 0.4 µM, simvastatin treatment resulted in a decrease in extracellular LDH levels, which serve as an indicator of cell rupture, by 47 and 57%, respectively, compared to the LDH levels observed in control cells (20). However, the levels of LDH release increased by 10% after treatment with 0.8 µM simvastatin (P<0.001; n=3) compared to the control group (Fig. 1B). This demonstrated that relatively low doses of simvastatin reduced cell damage, whereas slightly higher doses exacerbated it. Similar dose-dependent effects of simvastatin were observed with respect to Bax and Bcl-2 expression (Fig. 1A; P<0.05; n=3), indicating that higher doses of simvastatin (≥0.8 µM), as demonstrated in Fig. 1D, were associated with a pro-apoptotic expression pattern. The MTT assay showed that HUVEC viability decreased by 17, 32 and 65% after treatment with 1, 2 and 4 µM simvastatin, respectively, compared to that of untreated cells (Fig. 1C; P<0.001; n=6). Again, the expression levels of Bax and Bcl-2 proteins reflected the degree of cell damage at the same doses of simvastatin. Specifically, Bax expression increased by 26, 57 and 81%, and Bcl-2 expression decreased by 18, 33 and 60% after treatment with 1, 2 and 4 µM simvastatin, respectively (Fig. 1D; P<0.05; n=3). Since 1 µM simvastatin caused significant HUVEC injury, this concentration was used in the following experiments.
Figure 1.
Effect of simvastatin on HUVECs and its possible mechanisms. HUVECs were treated with simvastatin in two concentration series (0, 0.2, 0.4, and 0.8 µM; 0, 1, 2, and 4 µM) for 24 h. (A) Western blot analysis of the expression levels of Bax and Bcl-2 after exposure to simvastatin at 0-0.8 µM. (B) Assessment of endothelial dysfunction based on LDH release. (C) Cell viability tested by the MTT assay. (D) Western blot analysis of the expression levels of Bax and Bcl-2 after exposure to simvastatin at 0-4 µM. (E) Western blot analysis of the expression levels of β-catenin, phospho-β-catenin, GRP78 and ATF6. (F) The ratio of phospho-β-catenin/β-catenin. All values are presented as the mean ± SD; n=3 (in A, B, D, E and F) and n=6 (in C). *P<0.05 or **P<0.01 vs. respective 0 µM group. HUVEC, human umbilical vein endothelial cell; LDH, lactate dehydrogenase; phosphor, phosphorylated; GRP78, 78 kDa glucose-regulated protein; ATF6, cyclic AMP-dependent transcription factor ATF-6α.
Next, the effect of simvastatin on the Wnt/β-catenin pathway was examined by evaluating the expression of β-catenin and phospho-β-catenin, which are indicative of pathway activation and suppression, respectively (21-23). The β-catenin expression level was increased by 69, 115 and 146%, while the phospho-β-catenin level was decreased by 27, 43 and 59% after treatment with 1, 2 and 4 µM simvastatin, respectively (Fig. 1E, P<0.001; n=3). Thus, simvastatin promoted the activation of the Wnt/β-catenin pathway. Moreover, the protein expression levels of the ER stress-related markers GRP78 and ATF6 increased in a simvastatin dose-dependent manner, which confirmed that ER stress was also involved in simvastatin-induced ED (Fig. 1E; P<0.001; n=3). The ratio of phospho-β-catenin/total β-catenin was decreased along with the increase in the concentration of simvastatin. The ratio was decreased to 44, 24 and 15% of the control value after treatment with 1, 2, and 4 µM simvastatin (Fig. 1F; P<0.001; n=3).
Simvastatin promotes ED by activating the Wnt/β-catenin pathway
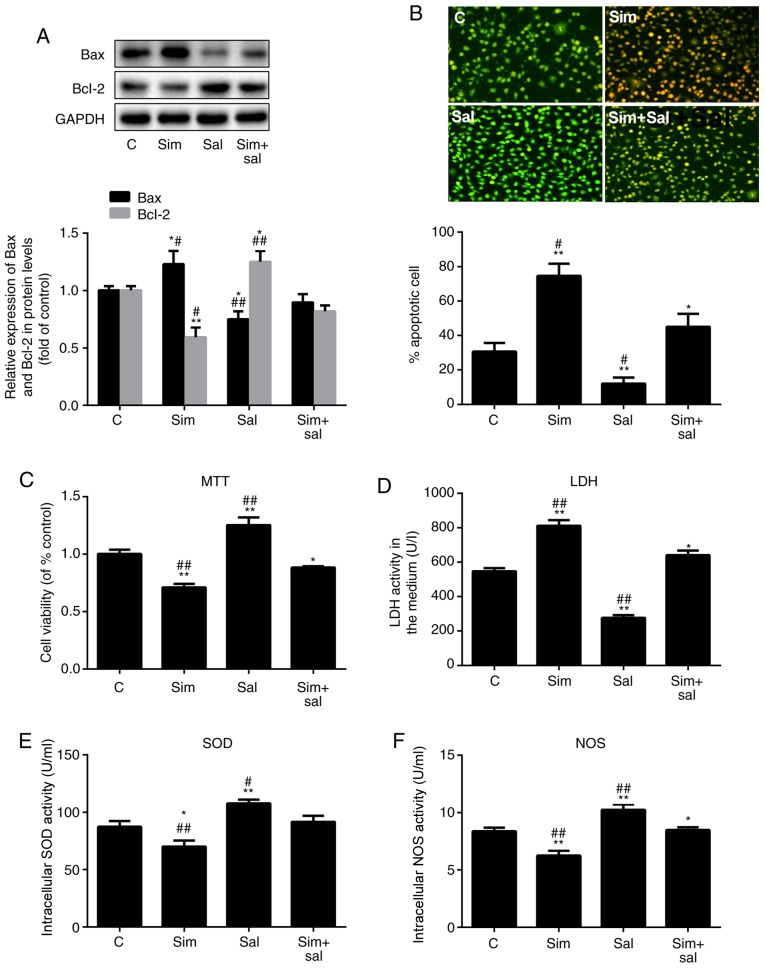
To verify whether simvastatin promoted HUVEC dysfunction through the Wnt/β-catenin pathway, HUVECs were treated with simvastatin, salinomycin or both for 24 h, and then exposed to H2O2 for an additional 24 h. Bax expression increased by 23% and decreased by 25% after treatment with simvastatin and salinomycin, respectively (Fig. 2A; P<0.05 and P<0.001; n=3). Compared to the combined treatment (Sim+Sal), simvastatin alone increased Bax expression by 37%, while salinomycin alone decreased the Bax level by 16%. Bcl-2 expression showed the opposite pattern of change (Fig. 2A; P<0.05 or P<0.001; n=3). Furthermore, the apoptosis rate HUVECs was measured by AO/EB staining. The results confirmed the same conclusion (Fig. 2B; P<0.05 or P0.001; n=3); compared with the control group, the apoptosis of HUVECS in the Sim group was significantly increased while that in the Sal group was significantly decreased.
Figure 2.
Simvastatin promotes ED by inducing the Wnt/β-catenin pathway. (A) Western blot analysis of Bax and Bcl-2 protein levels. (B) Acridine orange/ethidium bromide staining of human umbilical vein endothelial cells; magnification, ×20. The ED level of cells was reflected by the (C) MTT assay, (D) LDH levels, (E) SOD activity and (F) NOS activity. All values are presented as the mean ± SD. *P<0.05, **P<0.01 vs. C; #P<0.05, ##P<0.01 vs. Sim + Sal. n=3 (in A, B, D, E and F) and n=6 (in C). Sim, simvastatin; Sal, salinomycin; LDH, lactate dehydrogenase; ED, endothelial dysfunction; NOS, nitric oxide synthase; SOD, superoxide dismutase; C, control.
The effects of simvastatin on cell viability were also reversed by salinomycin (Fig. 2C; P<0.05 or P<0.001; n=6). Notably, the LDH leakage increased by 45% after treatment with simvastatin and decreased by 49% after exposure to salinomycin compared to that of control cells (Fig. 2D; P<0.05 or P<0.001; n=3). The effects of simvastatin and salinomycin on the intracellular levels of SOD (Fig. 2E; P<0.05 or P<0.001; n=3) and NOS (Fig. 2F; P<0.05 or P<0.001; n=3) were consistent with the aforementioned results. Therefore, these results indicated that 1 µM simvastatin promoted HUVEC apoptosis and inhibited cell viability by activating the Wnt/β-catenin pathway.
Simvastatin increases HUVEC adhesion ability by activating the Wnt/β-catenin pathway
HUVEC adhesion ability to THP-1 cells may reflect the degree of ED. To investigate the effect of the experimental conditions on HUVEC adhesion ability, the number of THP-1 cells adhering to HUVEC and the expression of adhesion molecules were measured. As shown in Fig. 3A, the number of HUVEC-attached THP-1 cells was increased by 47% and decreased by 22% after treatment with simvastatin and salinomycin, respectively, compared to control cells (P<0.001; n=3). Moreover, treatment with simvastatin and salinomycin alone resulted in a 32% increase and a 31% decrease in the number of HUVEC-attached THP-1 cells, respectively, compared to the combined treatment (P<0.001; n=3). Consistent results were obtained in terms of the protein levels of the adhesion markers VCAM-1, ICAM-1 and MCP-1, which were increased by 43, 17 and 26%, respectively, after treatment with simvastatin, and were decreased by 31, 38 and 17%, respectively, after exposure to salinomycin, compared to those of control cells (Fig. 3B; P<0.05 or P<0.001; n=3). Collectively, these results suggest that simvastatin promoted the adhesion ability of HUVECs through the Wnt/β-catenin pathway.
Figure 3.
Simvastatin increases the adhesion ability of HUVECs by inducing the Wnt/β-catenin pathway. (A) Adhesion rate of HUVECs to THP-1 cells; magnification, ×20. (B) Western blot analysis of VCAM-1, ICAM-1 and MCP-1 protein levels (mean ± SD). *P<0.05, **P<0.01 vs. C; #P<0.05, ##P<0.01 vs. Sim + Sal. n=3. HUVEC, human umbilical vein endothelial cell; VCAM-1, vascular cell adhesion protein 1; ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; C, control; Sim, simvastatin; Sal, salinomycin.
Simvastatin activates the Wnt/β-catenin pathway by enhancing LRP6 phosphorylation
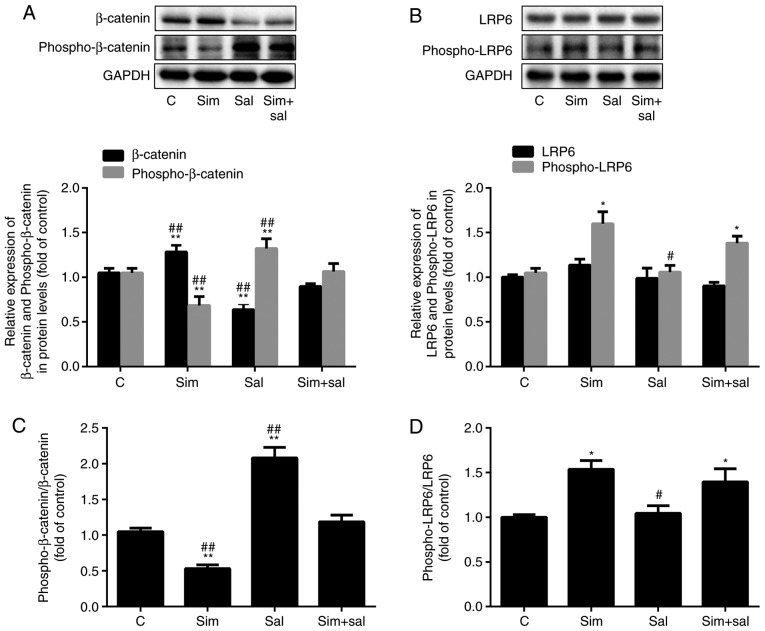
The levels of β-catenin were elevated after treatment with simvastatin and were attenuated by salinomycin (Fig. 4A; P<0.001; n=3). Specifically, β-catenin protein expression was increased by 22% and decreased by 40% after treatment with simvastatin and salinomycin, respectively. In addition, simvastatin decreased the level of phospho-β-catenin by 35%, while salinomycin treatment increased the phospho-β-catenin level by 26% compared to that of control cells. Compared to cells receiving the combined treatment, the phospho-β-catenin content was 36% lower in cells treated with simvastatin alone and was 24% higher in those exposed to salinomycin alone. Analysis of the ratio of phospho-β-catenin/total β-catenin produced similar results. As the ratio decreased by 53% after treatment with simvastatin and increased by 90% with salinomycin compared to that of control cells (Fig. 4B; P<0.001; n=3). The combined treatment mitigated the effects of simvastatin and salinomycin, as there was no statistical difference compared to the control group. Simvastatin affects cholesterol synthesis. Cholesterol is an important component of lipid rafts and the phosphorylation of LRP6, an upstream element of the Wnt/β-catenin pathway, is affected by lipid rafts (24). Notably, the expression level of phospho-LRP6 was increased by 52% after treatment with simvastatin (Fig. 4C; P<0.05; n=3), whereas no significant differences were observed in total LRP6 expression. However, the ratio of phospho-LRP6/LRP6 changed significantly. Specifically, the ratio increased by 54 and 39% after treatment with simvastatin and the combined treatment, respectively, compared to the control cells (Fig. 4D; P<0.05 or P<0.001; n=3).
Figure 4.
Simvastatin activates the Wnt/β-catenin pathway by enhancing LRP6 phosphorylation. The protein levels of (A) β-catenin, phospho-β-catenin, (B) LRP6 and phospho-LRP6 were measured by western blotting (mean ± SD). (C) The ratio of phospho-β-catenin/β-catenin and (D) phospho-LRP6/LRP6. *P<0.05, **P<0.01 vs. C; #P<0.05, ##P<0.01 vs. Sim + Sal. n=3. LRP6, low-density lipoprotein receptor-related protein 6; phospho, phosphorylated; Sim, simvas-tatin; Sal, salinomycin; C, control.
Simvastatin augments ER stress by activating the Wnt/β-catenin pathway
To verify the impact of simvastatin on ER stress, the expression of relevant protein markers was evaluated. Specifically, simvastatin increased the expression of the ER stress-related proteins GRP78, ATF6 and CHOP by 31, 33 and 24%, respectively, while salinomycin reduced the levels of these proteins by 25, 25 and 45%, respectively, compared to those of control cells. As expected, the combined treatment with simvastatin and salinomycin resulted in higher levels of ER stress markers than those observed with salinomycin treatment alone, but lower levels than those observed with simvastatin treatment alone (Fig. 5; P<0.05 or P<0.001; n=3). These findings suggested that simvastatin promoted ER stress via the Wnt/β-catenin pathway.
Figure 5.
Simvastatin augments endoplasmic reticulum stress through activation of the Wnt/β-catenin pathway. The protein expression levels of GRP78, ATF6 and CHOP were measured by western blotting (mean ± SD). *P<0.05, **P<0.01 vs. respective C; #P<0.05, ##P<0.01 vs. respective Sim + Sal. n=3. GRP78, 78 kDa glucose-regulated protein; ATF6, cyclic AMP-dependent transcription factor ATF-6α; Sim, simvastatin; Sal, salinomycin; C, control; CHOP, C/EBP-homologous protein.
Discussion
Simvastatin, a commonly used lipid-lowering drug, plays an anti-atherosclerotic role by reducing the level of blood lipids (25). However, its effects on endothelial cells under oxidative stress conditions and the possible underlying mechanisms are still unclear. The results of the present study demonstrated that when the concentration of simvastatin was <0.8 µM, H2O2-induced endothelial dysfunction was significantly reduced (Fig. 1A and B). However, at 1 µM, simvastatin significantly enhanced ED (Fig. 1C and D) and increased the β-catenin/phospho-β-catenin ratio, as well as the expression of ER stress markers (Fig. 1E and F). Therefore, it may be suggested that relatively high doses of simvastatin (≥1 µM) may induce ED by activating the Wnt/β-catenin pathway and ER stress.
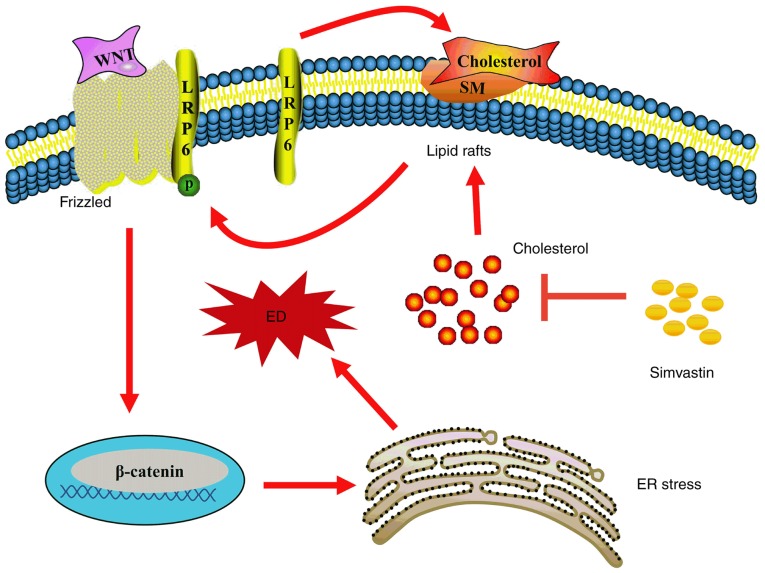
In the canonical Wnt/β-catenin pathway, LRP6 is phosphorylated and combines with Wnt molecules and Frizzled on the lipid raft, then delivers the signal downstream, thereby recruiting Dishevelled homolog proteins under the membrane and facilitating the phosphorylation of glycogen synthase kinase 3β; this results in large-scale accumulation of β-catenin (24,26). Lipid rafts are also termed microdomains, and are mainly anchored by cholesterol and sphingolipids (27); they can affect the localization of numerous receptors and signalling proteins, and therefore have a strong influence on multiple signal transduction cascades. For instance, lipid rafts play a vital role in lipopolysaccharide receptor (Toll-like receptor 4) and transforming growth factor-β receptor-mediated signal transduction (28,29). Activation of the Wnt/β-catenin pathway is facilitated by the localization of LRP6 in lipid rafts (30-32). However, methyl-β-cyclodextrin leads to the downregulation of LRP6 and β-catenin expression by depleting cholesterol and disrupting the structure of lipid rafts (31). Conversely, cholesterol supplementation upregulates the expression of LRP6 and β-catenin (33). In fact, in our previous study, 0.2 µM simvastatin had no effect on the expression or phosphorylation of LRP6, but reduced intracellular cholesterol deposition and inhibited ER stress (34). However, in the present study, 1 µM simvastatin was used to inhibit cholesterol biosynthesis, increase LRP6 phosphorylation, and activate the Wnt/β-catenin pathway by decreasing β-catenin phosphorylation and degradation (Fig. 4). Consequently, the accumulated functional β-catenin in the cytoplasm may be translocated to the nucleus and activate transcription factors such as those in the TCF/lymphoid enhancer-binding factor (LEF) family (35). Importantly, salinomycin, an inhibitor of the Wnt/β-catenin pathway, reversed these simvastatin-induced changes (Fig. 4). It may be inferred that simvastatin was able to regulate the Wnt/β-catenin pathway by affecting the phosphorylation of LRP6 on lipid rafts (Fig. 6).
Figure 6.
Proposed mechanism of action of simvastatin in promoting ED under oxidative stress. Simvastatin facilitates LRP6 phosphorylation and subsequent activation of the Wnt/β-catenin pathway, which promotes downstream ER stress, thereby leading to H2O2-induced ED. LRP6, low-density lipoprotein receptor-related protein 6; ED, endothelial dysfunction; ER, endoplasmic reticulum; SM, sphingomyelin; P, phosphate.
The involvement of the Wnt/β-catenin pathway in ED has also been demonstrated in previous studies (10,36,37). Treatment of HUVECs with 1 µM simvastatin led to a significant decrease in the intracellular activities of SOD and NOS (Fig. 2E and F). In addition, cell integrity was markedly destroyed, as demonstrated by the significant increase in the release of LDH into the medium (Fig. 2D). Furthermore, the decreased cell viability, as demonstrated in Fig. 2C, and changes in the expression of the apoptosis-related proteins (Bax and Bcl-2) indicated that 1 µM simvastatin can exacerbate the injury to HUVECs under oxidative stress conditions (Fig. 2A). The result of AO/EB staining also revealed that simvastatin was able to significantly promote the apoptosis of HUVECS under oxidative stress (Fig. 2B). In addition, this concentration of simvastatin clearly increased the number of THP-1 cells adhering to HUVECs, as well as the expression of adhesion molecules VCAM-1, ICAM-1 and MCP-1 (Fig. 3A and B). Salinomycin was able to reverse all these effects. These findings collectively demonstrated that high-dose simvastatin enhanced H2O2-mediated ED via activation of the Wnt/β-catenin pathway and the promotion of ER stress.
ER stress is characterized by the altered morphology and impaired function of the ER, and results from endogenous or exogenous stimuli that may lead to the accumulation of misfolded proteins in the ER. The main markers of ER stress are increased expression levels of GRP78, CHOP and ATF6 (12). Moreover, previous studies have shown that the Wnt/β-catenin pathway is able to regulate ER stress (32,38). For instance, inhibition of this pathway was shown to induce ER stress, leading to apoptosis in cancer cells (38). Furthermore, Zhang et al (39) proposed that inhibition of the Wnt/β-catenin pathway could cause ATF6-related ER stress in preadipocytes by promoting β-catenin degradation and leading to the inhibition of ATF6 functions, which are regulated by transcription factor LEF1, thus indicating a negative association between the Wnt/β-catenin pathway and ER stress. By contrast, the present study showed that in HUVECs, activation of the Wnt/β-catenin pathway induced by high-dose simvastatin was positively correlated with ER stress (Fig. 5). Furthermore, inhibition of the Wnt/β-catenin pathway has been demonstrated to promote apoptosis (38), whereas activation of this pathway has been shown to contribute to injury in HUVECs (10,26,37). Thus, the Wnt/β-catenin pathway may be closely associated with endothelial cell dysfunction, and its involvement in ER stress is plausible.
Many reports believe that classical pathways, such as the PI3K/Akt, isoprenoid and small G protein pathways, are protective to endothelial cells. Therefore, further investigation of these pathways may provide a greater understanding of the results of the present study. Furthermore, animal experiments or clinical studies may contribute further knowledge in relation to the mechanism (34,40,41). Overall, the present results revealed a novel, alternative mechanism of action of simvastatin, demonstrating that it can promote LRP6 phosphorylation, thereby activating the Wnt/β-catenin pathway and promoting ER stress, ultimately enhancing H2O2-induced HUVEC dysfunction (Fig. 6). In conclusion, this finding highlights the potential toxicity to endothelial cells of simvastatin at high concentrations, as well as its possible mechanism, providing a theoretical basis for better clinical control of the drug concentration and prevention of the potential toxicity and side effects of simvastatin.
Acknowledgments
Not applicable.
Abbreviations
- AS
atherosclerosis
- ED
endothelial dysfunction
- ER
endoplasmic reticulum
- FBS
foetal bovine serum
- HUVECs
human umbilical vascular endothelial cells
- LDH
lactate dehydrogenase
- MDA
malondialdehyde
- NOS
nitric oxide synthase
- SOD
superoxide dismutase
- TBST
Tris-buffered saline with 0.05% Tween-20
- AO
acridine orange
- EB
ethidium bromide
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81560151) and the Jiangxi Provincial Department of Science and Technology (grant no. 20181BAB205022).
Availability of data and materials
The analysed datasets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
NY conceived the study and designed the experiments. ZH and XD performed the experiments and data analysis. YW, LH, and LW made substantial contributions to interpretation of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Rapid progression of coronary atherosclerosis: A review. Thrombosis. 2015;2015:634983. doi: 10.1155/2015/634983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr GE, Young JC, Horvay K, Abud HE, Loveland KL. Regulated Wnt/beta-catenin signaling sustains adult spermatogenesis in mice. Biol Reprod. 2014;90:3. doi: 10.1095/biolreprod.112.105809. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31:2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011;31:1625–1633. doi: 10.1161/ATVBAHA.111.227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 8.Tsaousi A, Mill C, George SJ. The Wnt pathways in vascular disease: Lessons from vascular development. Curr Opin Lipidol. 2011;22:350–357. doi: 10.1097/MOL.0b013e32834aa701. [DOI] [PubMed] [Google Scholar]
- 9.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Hua L, Hou H, Du X, He Z, Liu M, Hu X, Yan N. Sphingomyelin synthase 2 promotes H2O2-induced endothelial dysfunction by activating the Wnt/β-catenin signaling pathway. Int J Mol Med. 2018;42:3344–3354. doi: 10.3892/ijmm.2018.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinozaki S, Chiba T, Kokame K, Miyata T, Kaneko E, Shimokado K. A deficiency of Herp, an endoplasmic reticulum stress protein, suppresses atherosclerosis in ApoE knockout mice by attenuating inflammatory responses. PLoS One. 2013;8:e75249. doi: 10.1371/journal.pone.0075249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic Biol Med. 2015;78:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Huang A, Patel S, McAlpine CS, Werstuck GH. The role of endoplasmic reticulum stress-glycogen synthase kinase-3 signaling in atherogenesis. Int J Mol Sci. 2018;19:E1607. doi: 10.3390/ijms19061607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halleskog C, Mulder J, Dahlström J, Mackie K, Schulte G, Hortobágyi T, Tanila H, Kumar Puli L, Färber K, Harkany T. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amodio G, Moltedo O, Faraonio R, Remondelli P. Targeting the endoplasmic reticulum unfolded protein response to counteract the oxidative stress-induced endothelial dysfunction. Oxid Med Cell Longev. 2018;2018:4946289. doi: 10.1155/2018/4946289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J, Kim K, Park E, Lee J, Markofski MM, Marrelli SP, Park Y. Exercise ameliorates endoplasmic reticulum stress-mediated vascular dysfunction in mesenteric arteries in atherosclerosis. Sci Rep. 2018;8:7938. doi: 10.1038/s41598-018-26188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garshick M, Underberg JA. The use of primary prevention statin therapy in those predisposed to atherosclerosis. Curr Atheroscler Rep. 2017;19:48. doi: 10.1007/s11883-017-0685-7. [DOI] [PubMed] [Google Scholar]
- 18.Gao K, Shen Z, Yuan Y, Han D, Song C, Guo Y, Mei X. Simvastatin inhibits neural cell apoptosis and promotes loco-motor recovery via activation of Wnt/β-catenin signaling pathway after spinal cord injury. J Neurochem. 2016;138:139–149. doi: 10.1111/jnc.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robin NC, Agoston Z, Biechele TL, James RG, Berndt JD, Moon RT. Simvastatin promotes adult hippocampal neurogenesis by enhancing Wnt/β-catenin signaling. Stem Cell Reports. 2013;2:9–17. doi: 10.1016/j.stemcr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan J, Yu Y, Li Y, Yu Y, Li Y, Zhou X, Huang P, Sun Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS One. 2013;8:e62087. doi: 10.1371/journal.pone.0062087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Qin X, Li P, Zhang H, Lin T, Miao Z, Ma S. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3β/β-catenin pathway in colorectal cancer cells. Drug Des Devel Ther. 2019;13:1449–1460. doi: 10.2147/DDDT.S192681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang LR, Kim SH, Baek SS. Effects of treadmill exercise on the anxiety-like behavior through modulation of GSK3β/β-catenin signaling in the maternal separation rat pup. J Exerc Rehabil. 2019;15:206–212. doi: 10.12965/jer.1938094.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvetorp A, Olsen RS, Nyström H, Skarstedt M, Dienus O, Mrowietz U, Söderman J, Seifert O. Expression of low-density lipoprotein-related receptors 5 and 6 (LRP5/6) in psoriasis skin. Exp Dermatol. 2017;26:1033–1038. doi: 10.1111/exd.13362. [DOI] [PubMed] [Google Scholar]
- 25.Harisa GI, Alomrani AH, Badran MM. Simvastatin-loaded nanostructured lipid carriers attenuate the atherogenic risk of erythrocytes in hyperlipidemic rats. Eur J Pharm Sci. 2017;96:62–71. doi: 10.1016/j.ejps.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Yan N, Li Z, Hua Y, Chen W. FGF19 sustains the high proliferative ability of keratinocytes in psoriasis through the regulation of Wnt/GSK-3β/β-catenin signaling via FGFR4. Clin Exp Pharmacol Physiol. 2019;46:761–769. doi: 10.1111/1440-1681.13103. [DOI] [PubMed] [Google Scholar]
- 27.Chung CL, Wang SW, Sun WC, Shu CW, Kao YC, Shiao MS, Chen CL. Sorafenib suppresses TGF-β responses by inducing caveolae/lipid raft-mediated internalization/degradation of cell-surface type II TGF-β receptors: Implications in development of effective adjunctive therapy for hepatocellular carcinoma. Biochem Pharmacol. 2018;154:39–53. doi: 10.1016/j.bcp.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Özhan G, Sezgin E, Wehner D, Pfister AS, Kühl SJ, Kagermeier- Schenk B, Kühl M, Schwille P, Weidinger G. Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev Cell. 2013;26:331–345. doi: 10.1016/j.devcel.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Yang Y, Gao Y, Wu X, Yang X, Zhu Y, Yang H, Wu L, Yang C, Song L. Elevated expression of flotillin-1 is associated with lymph node metastasis and poor prognosis in early-stage cervical cancer. Am J Cancer Res. 2015;6:38–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Haack F, Lemcke H, Ewald R, Rharass T, Uhrmacher AM. Spatio-temporal model of endogenous ROS and raft-dependent WNT/beta-catenin signaling driving cell fate commitment in human neural progenitor cells. PLoS Comput Biol. 2015;11:e1004106. doi: 10.1371/journal.pcbi.1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badana AK, Chintala M, Gavara MM, Naik S, Kumari S, Kappala VR, Iska BR, Malla RR. Lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Biomed Pharmacother. 2018;97:359–368. doi: 10.1016/j.biopha.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 32.Cao L, Lei H, Chang MZ, Liu ZQ, Bie XH. Down-regulation of 14-3-3β exerts anti-cancer effects through inducing ER stress in human glioma U87 cells: Involvement of CHOP-Wnt pathway. Biochem Biophys Res Commun. 2015;462:389–395. doi: 10.1016/j.bbrc.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Jia X, Chen Y, Zhao X, Lv C, Yan J. Oncolytic vaccinia virus inhibits human hepatocellular carcinoma MHCC97-H cell proliferation via endoplasmic reticulum stress, autophagy and Wnt pathways. J Gene Med. 2016;18:211–219. doi: 10.1002/jgm.2893. [DOI] [PubMed] [Google Scholar]
- 34.He Z, He X, Liu M, Hua L, Wang T, Liu Q, Chen L, Yan N. Simvastatin attenuates H2O2-induced endothelial cell dysfunction by reducing endoplasmic reticulum stress. Molecules. 2019;24:E1782. doi: 10.3390/molecules24091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H, Wang X, Liu K, Guo M, Zhang Y, Ying QL, Ye S. β-catenin coordinates with Jup and the TCF1/GATA6 axis to regulate human embryonic stem cell fate. Dev Biol. 2017;431:272–281. doi: 10.1016/j.ydbio.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, Song K, Lee I. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 37.Vikram A, Kim YR, Kumar S, Naqvi A, Hoffman TA, Kumar A, Miller FJ, Jr, Kim CS, Irani K. Canonical Wnt signaling induces vascular endothelial dysfunction via p66 Shc -regulated reactive oxygen species. Arterioscler Thromb Vasc Biol. 2014;34:2301–2309. doi: 10.1161/ATVBAHA.114.304338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Font E, Felipe-Abrio I, Calabuig-Fariñas S, Ramos R, Terrasa J, Vögler O, Alemany R, Martín-Broto J, Obrador- Hevia A. Disruption of TCF/β-catenin binding impairs Wnt signaling and induces apoptosis in soft tissue sarcoma cells. Mol Cancer Ther. 2017;16:1166–1176. doi: 10.1158/1535-7163.MCT-16-0585. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Wu S, Muhammad S, Ren Q, Sun C. miR-103/107 promote ER stress mediated apoptosis via targeting the Wnt3a/β-catenin/ATF6 pathway in preadipocytes. J Lipid Res. 2018;59:843–853. doi: 10.1194/jlr.M082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Doses of rosuvastatin, atorvastatin and simvastatin that induce equal reductions in LDL-C and non-HDL-C: Results from the VOYAGER meta-analysis. Eur J Prev Cardiol. 2016;23:744–747. doi: 10.1177/2047487315598710. [DOI] [PubMed] [Google Scholar]
- 41.Barale C, Frascaroli C, Cavalot F, Russo I. Hypercholesterolemia impairs the Glucagon-like peptide 1 action on platelets: Effects of a lipid-lowering treatment with simvastatin. Thromb Res. 2019;180:74–85. doi: 10.1016/j.thromres.2019.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysed datasets generated during the study are available from the corresponding author on reasonable request.