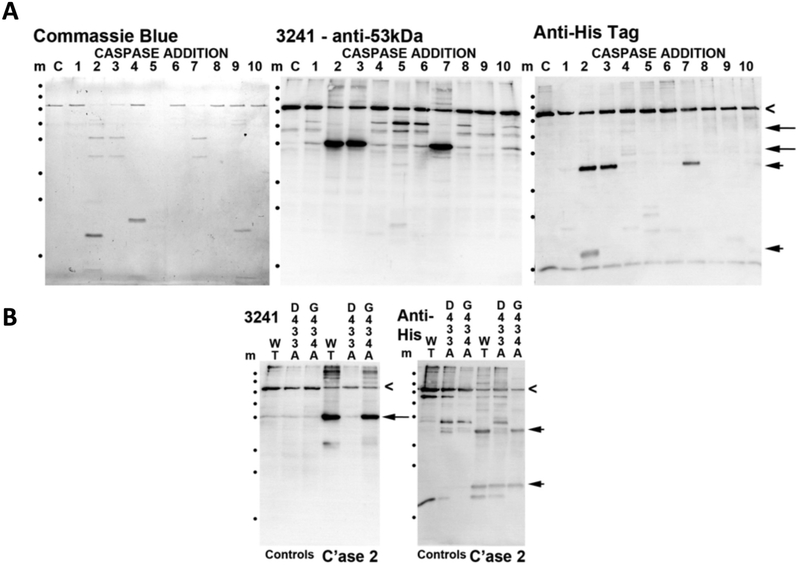
Fig. 2. Characterization of the caspase sensitivity of BFSP1.
A. In vitro cleavage of BFSP1 by purified caspases 1–10. Full length recombinant BFSP1 containing a C-terminal polyhistidine tag is indicated (chevron). Using the polyclonal antibody 3241 that was raised against the bovine 53 kDa fragment of BFSP1, two major fragments were detected (arrows). One corresponded to the 53 kDa fragment and like the slower migrating band of the two, both were undetected by the His-tag directed antibodies. Therefore both these fragments likely lack different proportions of the C-terminal sequences. Using the anti-His tag antibodies, two prominent bands were detected in the caspase 2 treated BFSP1 sample (arrowheads). Neither of these bands comigrated with bands detected by the 3241 polyclonal antibodies.
B. To evidence cleavage of BFSP1 by caspase 2 at the D433 and D549 sites, two BFSP1 mutants were produced, D433A and D549A. Comparing WT, D433A and D549A BFSP1 sensitivity to caspase 2 cleavage resulted in the absence of the characteristic 53 kDa fragment (arrowhead) in the G433A BFSP1 as detected by the 3241 polyclonal antibodies. Using the anti-His tag antibodies, two characteristic fragments (arrowheads) were detected in the wild type and D549A BFSP1 samples, but only the faster migrating band in the D433A BFSP1 sample. Similarly the bands indicated (Asterisks) were present in the WT and D433A BFSP1 samples but missing from the D549A BFSP1 sample. This indicates that the mutations have abolished one site at position D433 and one at D549 for caspase 2.