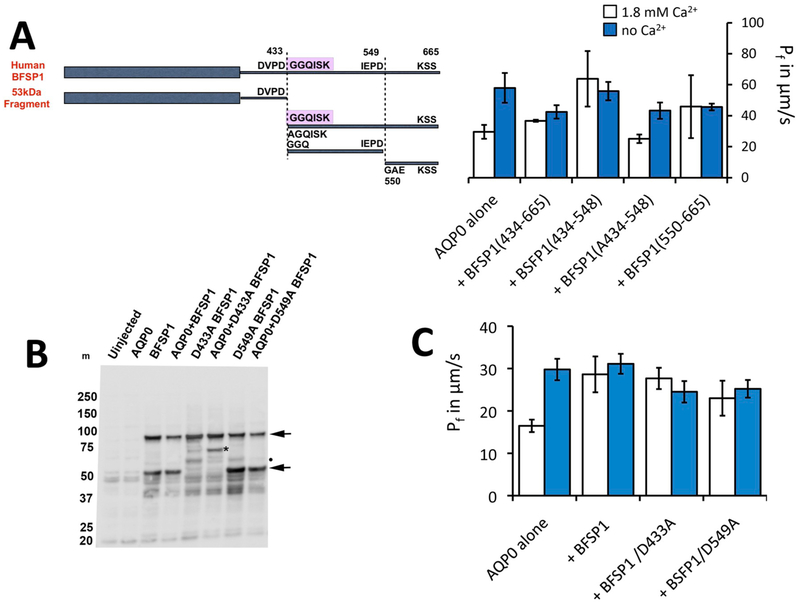
Fig. 6. Regulation of AQP0 by BFSP1 C-terminal domains.
A. Xenopus oocytes were injected with AQP0 alone or in combination with the indicated BFSP1 constructs. C-terminal constructs could be divided into two classes: ones which left the calcium response essentially identical to wild type, that is Pf was low in 1.8 mM Ca2+ and high in 0 mM Ca2+; or ones in which Pf did not change with Ca2+ concentration. Results were analyzed by omnibus ANOVA followed by pair wise t-tests for individual 0 mM Ca2+ - 1.8 mM Ca2+ comparisons. In the cases where Pf increased in response to no calcium, pairwise p values were less than 0.05 (*). In cases where Pf did not respond to changes in calcium, p values were greater than 0.3 (ns). Conditional p values for 1.8 mM Ca2+ vs no Ca2+: AQP0 alone = 0.0232, +434–665 = 0.0328, +434–548 = 0.6823, +A434–548 = 0.0128,
B. Immunoblots showing that BFSP1 was expressed in Xenopus oocytes when appropriate constructs were injected. Two major immunoreactive bands were detected (arrows), the faster migrating band a fragment derived by proteolytic cleavage because the D433E mutation in BFSP1 altered the pattern achieved. Notice too that the presence of AQP0 altered the banding pattern achieved with the D433A mutant (*). BFSP1 immunoreactive bands are found in the pellet fraction prepared from injected oocytes.
C. Full length BFSP1 and full length BFSP1 with mutations at putative caspase sites eliminated Pf regulation by calcium. Conditional p values for 1.8 mM Ca2+ vs no Ca2+: AQP0 alone = 0.001, +BFSP1 = 0.617, +BFSP1 D433A = 0.3913, +BFSP1 D549A = 0.6445.