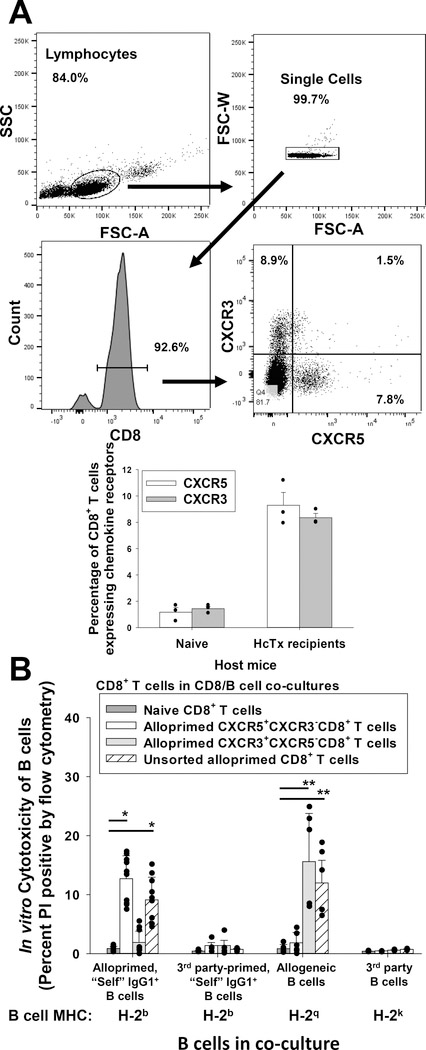
Figure 1. CXCR5+CD8+ T cells mediate in vitro cytotoxicity of alloprimed self IgG+ B cells.
C57BL/6 (wild-type, WT; H-2b) mice were transplanted with FVB/N (H-2q) hepatocytes. On day 7 post transplant, splenic CD8+ T cells were retrieved and purified. A) Flow cytometric analysis of purified CD8+ T cells shows that CXCR5+CXCR3− (9.3±1.0%) and CXCR3+CXCR5− (8.4±0.3%) CD8+ T cell subsets are detected (CD8+ T cells pooled from 10–15 mice) for cell sorting; data for n= 3 sorts are shown in the bar graph. B) In an in vitro cytotoxicity assay, flow-sorted alloprimed CXCR5+CXCR3− or CXCR3+CXCR5− CD8+ T cell populations and B cell targets were co-cultured at a 10:1 ratio for 4 hours and analyzed for cytotoxicity (propidium iodide (PI) uptake). Naïve CD8+ T cells and unsorted alloprimed CD8+ T cells were utilized as negative and positive controls (effector cells), respectively. Significant cytotoxicity of alloprimed, self IgG1+ B cells was observed in co-cultures with CXCR5+CXCR3−CD8+ T cells (12.7±1.8%, n=10) or with unsorted alloprimed CD8+ T cells (9.1±3.8, p<0.0001 for both signified by “*”; n=10) in comparison to co-cultures with naïve CD8+ T cells (0.9±0.4%, n=9) but no significant cytotoxicity was detected in co-cultures with CXCR3+CXCR5−CD8+ T cells (1.9±1.9%, p=ns, n=10). Significant cytotoxicity of allogeneic B cells was observed in co-cultures with CXCR3+CXCR5−CD8+ T cells (15.6±8.8%; n=6) or with unsorted alloprimed CD8+ T cells (12.0±5.5%; n=6, p<0.002 for both signified by “**”), but not with CXCR5+CXCR3−CD8+ T cells (1.8±1.8%; n=6, p=ns) in comparison to control co-cultures with naïve CD8+ T cells (0.8±0.7%; n=5). No significant cytotoxicity was detected against third-party (3rd) party primed “self” IgG1+ B cells (H-2b targets) or 3rd party B cells (H-2k targets) in any co-cultures. Error bars indicate standard error from triplicate experiments.