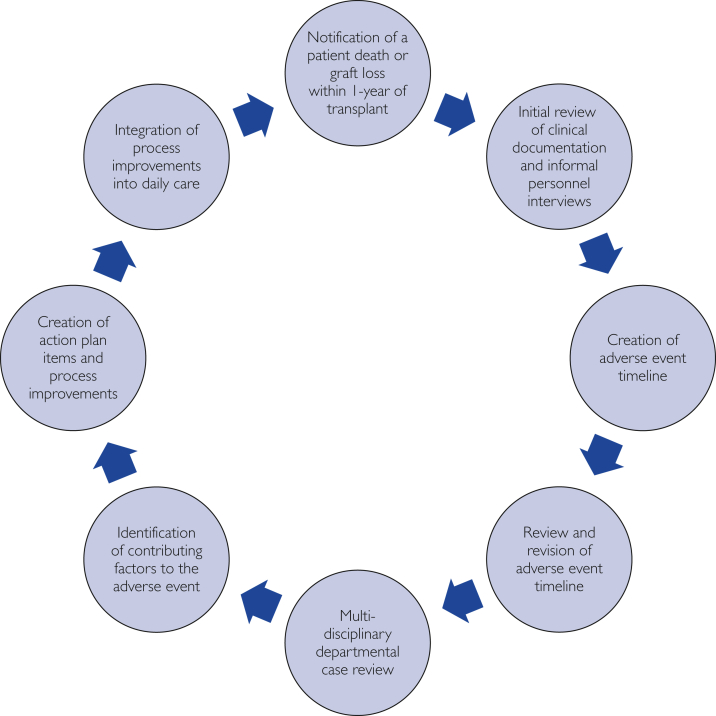
Figure 1.
The departmental case review process for adverse-event review. The review of adverse events within 1 year of transplant follows a precisely structured iterative process. The process begins with the occurrence and notification of an adverse event, including patient deaths, graft losses, and other patient-safety events. This process includes exhaustive narrative timeline review, multidisciplinary discussion, and creation of action-plan items directed to process improvement, using quality improvement methods.