Figure 2.
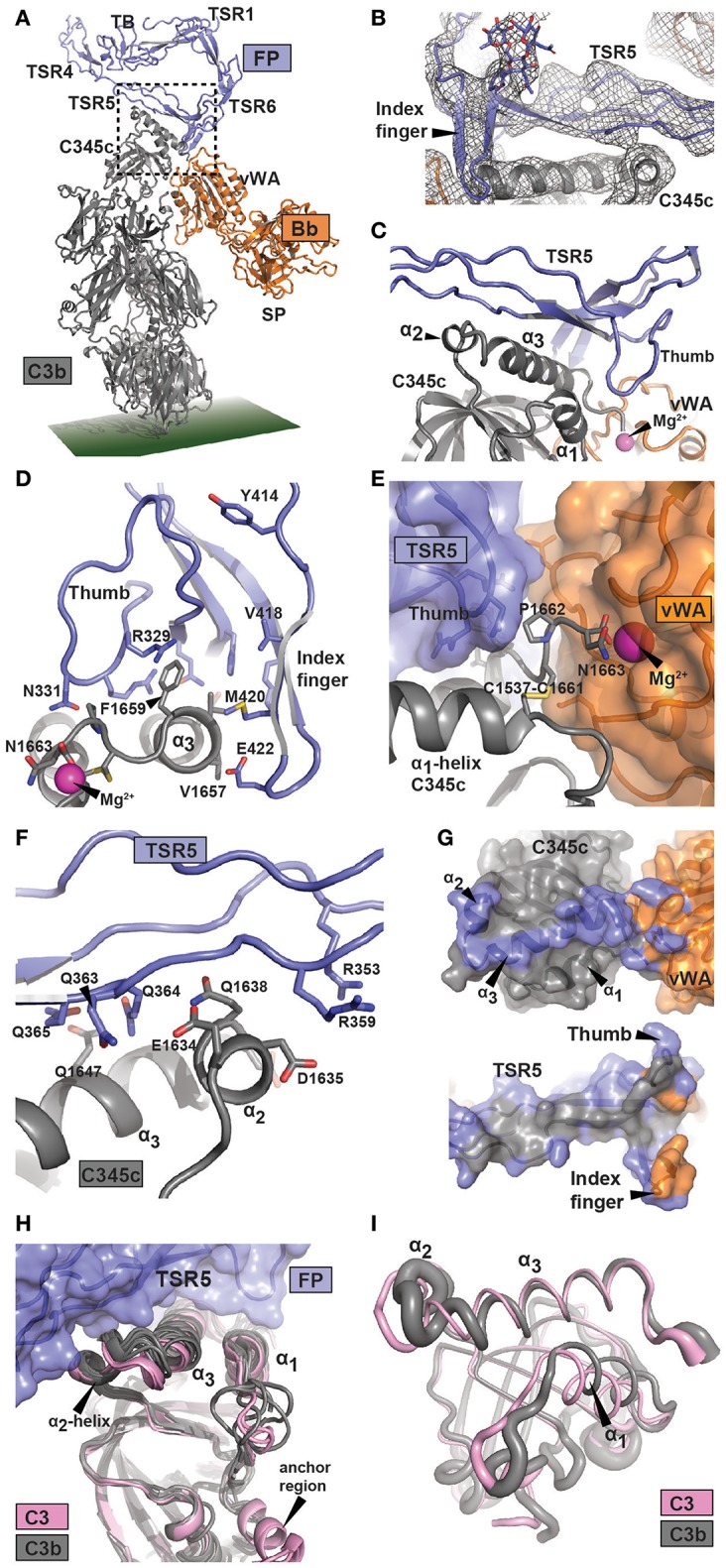
Structure of the FP bound AP C3 convertase. (A) Outline of the FP bound convertase shown in a cartoon representation. In FP only the TB domain, TSR1 and TSR4-6 were modeled. The SCIN protein used to stabilize C3bBb is not shown. (B) A 2mFo-DFc omit map contoured at 1σ in which FP TSR5, the FP TSR6 index finger, and all associated glycosylations were omitted from map calculation. Unambiguous density is observed for the index finger and its Asn-linked glycan. (C) Close-up of the region outlined in panel A with a dotted rectangle containing the FP-C3b interface. The α2- and α3-helices and their connecting loop in the C3b C345c domain are recognized by the concave face of FP TSR5. (D–F) Details of the FP-C3 convertase interface with selected residues shown as sticks. (D) Outline of the interaction between the thumb and index finger of TSR5 and TSR6, respectively, and the α3-helix of C3 C345c. The C-terminal of C3 C345c protruding from the α3-helix completes the coordination of the Mg2+-ion present in the MIDAS of Bb. (E) The Bb vWA-C3 C345c interface, suggesting the importance of the FP TSR5 thumb in stabilizing the interaction. (F) The interaction surface formed between TSR5 and the C3 C345c α2 and α3 helix. A large number of basic residues in TSR5 are presumably interacting with the acidic α2 helix in C3b. (G) Footprint of FP on the AP C3 convertase (Top) or AP C3 convertase on FP (bottom). The footprint of FP on C3b and Bb is colored blue, whereas the footprint of C3b and Bb on FP is colored gray and orange, respectively. (H) Superposition of six C3b structures (gray) and two C3 (pink) structures on the central β-sheet of the C3 C345c domain. This demonstrate a clear difference around the α2-helix which may explain the specificity of FP for C3b. (I) Residue fluctuations of the C345c domain of C3b (gray) and C3 (pink) during molecular dynamics simulations. The thickness of the residues is proportional to the root mean square fluctuations in a 1 μs simulation.
