Abstract
Progression of renal parenchyma injury is characterized by increasing interstitial fibrosis and tubular atrophy, irrespective of the cause. Histopathologic assessment of renal tissue obtained by biopsy remains the gold standard for determining the presence and extent of tubulointerstitial scarring. Discovery of robust non-invasive means for capturing a snapshot and for longitudinal monitoring of parenchymal deterioration has been the focus of intense multimodal effort by investigators within the renal community and beyond. Research in this field has included the use of in vitro and in vivo experimental models and has fostered the development and evaluation of tissue and biofluid assays for novel analytes with potential translation to the diagnosis and prognosis of kidney disease. Here, we examine recent advances in the search of “biomarkers” for detection of renal tubulointerstitial scarring and prediction of renal outcome in human renal disease.
Keywords: biomarker, biopsy, chronic kidney disease, extracellular matrix, fibrosis, kidney, transforming growth factor beta
Introduction
Normal renal parenchyma is a structurally diverse tissue including glomerular, tubulointerstitial, and vascular compartments. Scarring due to injury or aging can occur in all of these structures, separately or in concert, manifested histopathologically as glomerulosclerosis, interstitial fibrosis (IF), tubular atrophy (TA), and arteriosclerosis. Tubulointerstitial scarring (IF/TA) is a composite of IF and TA, and these features are for the most part synchronous. IF/TA is an important determinant of irreversible progression in different types of injury affecting native and transplanted kidneys. Technical advances and new assays are coming to the fore at a rapid pace, augmenting and improving the accuracy of tissue-based diagnosis, with the added promise of finding new non-invasive “biomarkers” for detecting and staging kidney disease.
A “biomarker” can be defined as any characteristic that is measured as an indicator of a normal biological process, pathologic process, or response to an exposure or intervention, including a therapeutic intervention.1 Introduction of new candidate biomarkers for IF/TA should be based on strong evidence supporting their safety, high sensitivity and specificity, superiority, or at least equivalence with available non-invasive means for quantifying IF/TA, and ideally a biomarker should have predictive value for chronicity and progression of renal disease in human subjects.2 The purpose of this review was to discuss potential “biomarkers” of renal IF/TA, focusing on IF.
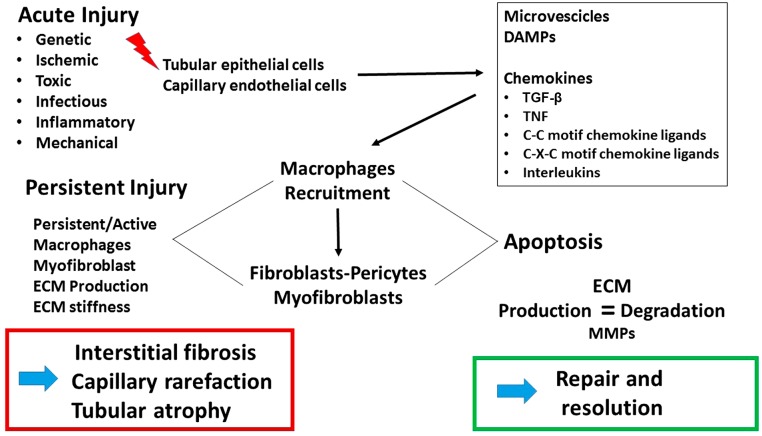
Development of IF and TA may ensue after a failed reparative response or persistence of a variety of injurious factors, including inflammation, ischemia, and toxins as the most common,3 involving several pathogenetic mechanisms (briefly illustrated in Fig. 1). An in-depth discussion on the pathogenesis of fibrosis is beyond the scope of this article, and numerous excellent reviews are available on this topic.4–8 As is common to many organs, following an insult to the renal parenchyma, myofibroblasts are activated and a multitude of pathways ultimately converge on transforming growth factor beta–mediated production of fibrillar collagens, especially collagens I and III. The source of myofibroblasts has been much debated, and several lineages have been proposed, including pericytes,9,10 native renal fibroblasts,11 epithelial mesenchymal transformation of tubular epithelium,12 endothelial–mesenchymal transformation,13 and transdifferentiation of monocytes recruited from bone marrow.14 Insights into the matricellular origin of fibrosis are key to deriving a tissue-specific biomarker for the maladaptive repair process observed as fibrosis. To this end, cell enumeration technologies such as single-cell RNA sequencing applied to human kidney biopsies15,16 may prove invaluable in mapping the lineage pathway to renal myofibroblast activation.
Figure 1.
Schematic illustration of steps in the development of renal interstitial fibrosis and tubular atrophy. Abbreviations: TGF-β, transforming growth factor beta; TNF, tumor necrosis factor; ECM, extracellular matrix; MMP, matrix metalloproteinases; DAMPs, damage-associated molecular patterns.
The Kidney Biopsy
The kidney biopsy remains central in establishing a diagnosis and prognostication of native kidney diseases and renal allograft dysfunction. Routine diagnostic evaluation of the presence and extent of pathologic IF and TA still relies mostly on visual inspection of renal tissue by light microscopy, immunofluorescence, immunohistochemistry, and electron microscopy. Frequently in the pathologic evaluation of chronic lesions in the kidney biopsy, the extent of IF is found to be very similar to the degree of TA; however, different conditions may affect predominantly one of the two compartments, as, for example, when tubular dilatation as a result of chronic obstruction is more extensive than interstitial fibrotic expansion.
Strong correlation between the degree of interstitial and tubular scarring and renal function has long been known and is supported by a large body of literature.17–20 The potential predictive value of IF/TA for renal disease progression is being increasingly recognized, and IF/TA scoring has been incorporated into pathologic guidelines for the classification of renal diseases such as the MEST score for IgA nephropathy,21 the Banff classification of transplant rejection,22 lupus nephritis,23,24 and diabetic nephropathy.25 Although most IF scoring in the above-mentioned classifications is semiquantitative, a precise determination of IF in kidney biopsies using a continuous score is desirable as shown by Mariani et al.26 In this study, cortical IF/TA was scored as a continuous percentage by visual estimate from whole slide digital images of kidney biopsies from 315 patients enrolled in the renal NEPTUNE trial. We demonstrated not only excellent inverse correlation with the estimated glomerular filtration rate (eGFR) (r = −0.7, p<0.001) at the time of biopsy but also predictive value for renal outcome, with each 10% increase in IF associated with a hazard ratio of 1.29 (p<0.03) for end-stage renal disease/40% of eGFR decline.26
Various ways to improve precision and reproducibility for measuring the degree of scarring on kidney biopsies by traditional light microscopy or digital microscopy have been described,27–30 and novel technical approaches have emerged for visual analysis of fibrosis in tissue samples. One example is second harmonic generation (SHG), a non-linear multiphoton imaging modality that exploits the physical-chemical characteristics of collagen. Fibrillary collagens (collagens I and III) have a triple-helix structure, extremely high level of crystallinity, and non-centrosymmetric structure that gives rise to detectable SHG of incident light very efficiently and specifically.31 SHG has been used to measure fibrosis in a label-free, species- and tissue-agnostic manner in mouse and humans kidneys.31–33 Although it has achieved broader reception for fibrosis metrics in liver biopsies for non-alcoholic fatty liver disease/steatohepatitis (NAFLD/NASH), it has only been superficially explored to quantify fibrotic interstitial expansion in the kidney.34 SHG can also be used for intravital imaging in living tissues in an endoscopic format.35 Although promising, SHG has only been used in experimental contexts and not adequately validated for diagnostic application to measure IF in kidney biopsy specimens.
Imaging
Non-invasive imaging techniques have been applied to the study of kidney fibrosis, including ultrasound elastography (UE), magnetic resonance imaging (MRI), and contrast-enhanced computed tomography (CT). UE can detect abnormal stiffness in an organ and can be performed with qualitative and quantitative modalities, among which transient elastography is well suited for measurements in superficial organs such as liver, breast, and thyroid and is routinely performed to assess the elastic properties of liver parenchyma in hepatic diseases. In the kidney, shear wave elasticity imaging has been the preferred approach; however, the deep anatomical location of the kidney, and its architectural complexity, represents a major challenge in the use of this techniques; definitions of normal range for kidney elasticity are still unavailable, and further improvements in the performance of this imaging approach in the native kidney are needed.36 The anatomical superficiality of the transplanted kidney makes it easier to access, potentially facilitating the application of UE to test allograft stiffness as an index of IF, by its mean organ stiffness, cortical stiffness, and corticomedullary strain ratio. Among UE techniques, transient elastography may be the most effective non-invasive imaging method to detect early allograft fibrosis, but its accuracy is not high enough to measure the extent of interstitial fibrotic changes.37
Some non-contrast MRI methods have been tested to detect IF/TA: one, diffusion-weighted imaging (DWI), is based on water molecules’ motion, and another, blood oxygenation level–dependent imaging, is based on the extent of reduced blood flow to the kidney. Main hurdles that currently impede using MRI to measure kidney fibrosis are the heterogeneity of kidney architecture, the complex fluid intraparenchymal compartmentalization, and its variability in physiologic and pathologic conditions.38,39
Contrast-enhanced CT imaging has been used to assess the quantification of renal relative blood volume as an index of renal capillary rarefaction, a feature of kidney fibrosis, in patients with normal renal function and in patients with chronic kidney disease (CKD) before nephrectomy.40 In this study, microvascular rarefaction was correlated with histological measurement of fibrosis and capillary density and with a decline in GFR. Although promising, the need of radiocontrast may prevent broader use of this approach.
Molecular Biomarker Discovery
Bioassays of compounds present in kidney tissue, urine, and blood have been explored in search for biomarkers of CKD. Changes in gene expression and molecules involved in profibrotic biological pathways have been measured and correlated to the degree of renal fibrosis, and biomolecules with a direct link to pathophysiological processes may be viable candidate biomarkers with clinical resonance.
Tissue
In most studies on human kidney biopsies, changes in tissue mRNA and protein expression have been evaluated as indicators of the degree of IF and in relation to eGFR at the time of biopsy. An added feature in tissue biomarker discovery should be search of biomarkers with value as predictors for progression and outcome.
Compared with healthy kidney tissue, progression of kidney lesions is accompanied by abnormal upregulation and downregulation of certain signaling pathways; prominent among the upregulated pathways are immune responses including antigen processing and presentation, major histocompatibility complex protein expression, T- and B-cell receptor signaling, complement pathway, defense responses, responses to wounding, regulation of cell proliferation, and regulation of extracellular matrix organization.5
Control of these processes involves mediators such as transforming growth factor beta 1 (TGF-β1), tumor necrosis factor (TNF), matrix metalloproteinases (MMPs), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and alteration of their downstream effectors in pathologic settings can lead to an increase in collagen synthesis and matrix protein accumulation, hallmarks of fibrotic states.41 A brief description of these mediators, as well as their utility individually or in a panel for study of kidney fibrosis, is provided below.
TGF-β1 is a growth factor with multiple functional effects through interactions with its canonical receptors as well as other growth factor pathways and has been the focus of many studies as a potential biomarker and therapeutic target for fibrosis in general and renal fibrosis in particular.42 TGF-β1 stimulates fibroblast proliferation and transformation into myofibroblasts, resulting in an increased production of α-smooth muscle actin and extracellular matrix (ECM) components, essential for the development of fibrosis.
Tumor necrosis factor-α (TNF-α) is a proinflammatory mediator, produced by T-lymphocytes, endothelial cells, and renal tubular epithelial cells. TNF-α is involved in inflammatory responses, reparative tissue responses, and cell regenerative and proliferative responses, and its secretion is increased during inflammatory states. MMPs play a major role in interstitial remodeling by cleaving various ECM proteins, as well as other substrates such as cadherins, integrins, TGF-β, and fibroblast growth factor receptor 1. MMPs are classified based on their substrate selectivity and are differentially expressed in glomeruli and nephron segments; their activity is inhibited by tissue inhibitors of metalloproteinases (TIMPs), also expressed in the kidney.43 A role for MMPs and TIMPs has been proposed in the progression of fibrosis in the kidney, and increased expression of both has been studied in chronic renal inflammation and/or fibrosis in the human kidney.
Epidermal growth factor (EGF) is the canonical ligand of the epidermal growth factor receptor (EGFR, ErbB1), a membrane tyrosine kinase receptor expressed in the kidney, which is activated after renal damage; other ligands for EGFR include transforming growth factor alpha, heparin-binding epidermal growth factor, and connective tissue growth factor, also known as CTGF/CCN2. Dysregulation of the EGFR pathway has been reported to have a role in the development of IF/TA in the kidney.
Each of these mediators has been proposed as a potential marker of fibrosis in human kidney biopsy tissue, and relevant studies are summarized below.
Increased expression of MMPs (MMP-3, MMP-7, MMP-9), interleukin (IL)-8, urokinase R, and integrin-β4 in 32 native biopsies from a European cohort was associated with fibrosis and progression during follow-up.44
In an analysis of the predictive value of gene transcript from kidney biopsies for eGFR at the time of biopsies in four separate cohorts of patients with various kidney diseases, EGF was the best predictor among six genes (nicotinamide N-methyltransferase [NNMT], thymosin b-10 [TMSB10], tissue inhibitor of metalloproteinases [TIMP1], tubulin a 1A [TUBA1A], and annexin A1 [ANXA1]).45 Intrarenal expression of EGF and its urinary excretion were also found to have significant inverse correlation with IF.45
Measurement of gene expression performed on microdissected tubulointerstitial tissue in 165 renal biopsies of 315 patients with proteinuric glomerulopathies enrolled in the NEPTUNE study revealed a high correlation between the degree of IF/TA and changes in the expression of profibrotic genes regulated by TNF, TGF-β1, interferon-γ, IL-1, and NF-kB, and decreased expression of EGF was also noted.26
Human transplanted kidneys have also been studied with similar results, even though most investigations have focused on markers of rejection. Quantification of mRNA expression in transplant biopsies, 10 with IF/TA and 5 control donor biopsies, showed increased tubulointerstitial expression of TGF-β, thrombospondin-1, fibronectin, and MMP-7 mRNA levels in samples with fibrosis compared with normal biopsies; normal donor biopsies showed increased EGF expression.46 Another study also reported inverse correlation of tissue EGF expression and IF/TA in transplanted kidney biopsies.47 Analysis of the differentially expressed gene in kidney allograft biopsies at 3 and 12 months showed upregulation of genes related to fibrosis such as collagen type I alpha 2 chain (COL1A2), decorin (DCN), and MMP-2 to occur in a late posttransplant phase.48 A predictive 13-gene transcript signature in allograft kidney biopsies for risk of progressive damage in kidney transplants after 1 year has been proposed49; unfortunately, only the Chronic Allograft Damage Index was used for scoring histology, and no direct quantification of fibrosis was provided.
Most of the above-cited studies confirm activation of gene pathways involved in matrix remodeling and immune-related mechanisms, with common upstream regulators, but fail to provide convincing rationale for the added value of measuring expression of these genes in kidney biopsy tissue to predict progression of fibrosis.
Blood and Urine
Many potential blood and urine biomarkers of IF and progression of chronic kidney damage have been described and recently reviewed.50,51 These include urinary MMP-7,52 urinary fibrinogen,53 and serum levels of soluble Klotho.54–56 (The Klotho soluble form α-Klotho can bind to TGF-β receptor type 2, inhibit TGF-β1, and also suppress the activity of Wnt.57) Measurement of mRNA of extracellular matrix components in the urine sediment had also been looked at as a potential biomarker of TF/IA, including the mRNA of collagen 1 A1 chain (COL1A1) and of fibronectin.58 Combinatorial approaches to aggregate these biomarkers are a ripe area for exploring novel panels for diagnosis and prognosis.
The available literature was reviewed by Mansour et al.50 to evaluate the reliability and performance of blood and urine biomarkers to identify IF on native or transplanted kidney biopsies, and their predictive value for renal outcome in the published literature. From 3681 published studies, nine biomarkers were deemed acceptable (i.e., correlation coefficient with fibrosis on renal biopsy r >0.40, or area under the curve >0.65). These are amino-terminal propeptide of type III procollagen (PIIINP; urine and blood), TGF-β (urine), monocyte chemoattractant protein-1 (MCP-1; urine); hepcidin (urine), liver-type fatty-acid-binding protein (LFABP; urine); plasminogen activator inhibitor 1 (PAI-1; blood), MMP-2 (urine), and TIMP-1 (urine). The highest biomarker performance for identifying patients at risk of fibrosis and worse renal outcome was assigned to urine TGF-β, MCP-1, and MMP-2.
Of note, MCP-1 has shown promise as an indicator of IF in the kidney of 634 living donors, with significant increase (p=0.0005) in urine MPC-1/creatinine (Cr) correlated with relatively mild levels of IF ranging from 5% to 10%.59
Although most of the biomarkers discussed to this point have focused on IF pathways, urinary EGF (uEGF) was recently described as the most promising biomarker of TA. As mentioned earlier, IF and TA are synchronous processes for the most part. Ju et al.45 showed high inverse correlation of uEGF with the percentage of cortical IF/TA measured in kidney biopsies of patients with proteinuria and also showed that uEGF protein/Cr was a predictor of eGFR slope (r = 0.65, p<0.001) in a multivariable regression model, confirming older reports that uEGF could be a marker of renal disease progression.60 Nowak et al.61 examined the prognostic value of serum and urinary markers as predictors of progressive renal function decline in 1032 subjects with type 2 diabetes and found that a decreased ratio of uEGF/MCP-1 had a high prognostic value. Azukaitis et al.62 examined uEGF/Cr in assessing the probability of progression in a cohort of 623 pediatric patients with CKD and found that lower values of uEGF/Cr were associated with higher risk of CKD progression. Thus, uEGF/Cr may prove to be a useful longitudinal biomarker to assess progressive renal parenchymal scarring.
MicroRNAs (miRNAs)
Small RNAs or miRNAs typically function as regulatory molecules of coding RNA species such as mRNAs and have been investigated as potential biomarkers in kidney disease. Below are some of the results from human studies, although additional miRNAs have been proposed from studies on model organisms.63,64 Among the most frequently reported miRNAs are miR-21, miR-29, and miR-93, which are downstream of TGF-β-Smad signaling; increased urinary levels of these molecules would be functionally consistent with the alteration of a key pathway driving fibrogenesis.64 In allografts, serum/plasma miR-145-5p, miR-148a, miR-150, miR-192, and miR-200b were inversely correlated with graft function and IF/TA, whereas upregulation of plasma/serum miR-423-3p, miR-21, miR-142-3p, and miR-155 and urinary miR-21 and miR-200b correlated with graft dysfunction.65–68 Meta-analysis of renal fibrosis studies identified five upregulated miRNAs (miR-142-3p, miR-223-3p, miR-21-5p, miR-142-5p, and miR-214-3p) and two downregulated miRNAs (miR-29c-3p and miR-200a-3p) as possible biomarkers of fibrosis.69 In diabetic kidney disease, a review of miRNA dysregulation identified miR-21-5p, miR-29a-3p, miR-126-3p, miR-192-5p, miR-214-3p, and miR-342-3p as possible biomarkers.70 In IgA nephropathy, urinary sediment miRNAs have been assessed, and significantly lower urinary miR-34a, miR-205, and miR-155, but higher miR-21 were appreciated.71 Urinary miR-196a was reported to distinguish focal segmental glomerulosclerosis patients with complete remission from those with nephrotic-range proteinuria, as well as predicting IF/TA.
Although miRNAs hold promise as biomarkers, their lack of tissue specificity, oftentimes lack of functional implications, and few reproducibility studies cast doubts on their value, and their current status as a clinically meaningful biomarker is not established.
Exosomes and Microvesicles
A few studies have examined the content of urine exosomes as a possible source of biomarkers for kidney fibrosis. Urinary exosomes are small microvesicles ranging in size from 30 to 100 nm; in the kidney, they originate from glomerular and tubular cells and can be isolated in small amount from urine typically through ultracentrifugation.72
Analysis of mRNA transcripts in urinary exosomes collected from 32 subjects undergoing kidney biopsies compared with non-biopsied healthy controls showed a low but significant correlation (r = 0.394, p=0.03) with the percentage of IF and with eGFR of CD2-associated protein (CD2AP). Mutations of this gene have been associated with focal segmental glomerulosclerosis and nephrotic syndrome, but the pathophysiologic context for CD2AP as a as biomarker of fibrosis is not clear.73
Urinary levels of endothelial microparticles (EMPs) as a possible marker of decreased density of peritubular capillaries and fibrosis were examined in 38 hypertensive patients, of which 7 had kidney biopsies, and in 14 non-biopsied controls. Peritubular capillary (PTC)-EMPs were identified as urinary exosomes positive for the plasmalemmal-vesicle-associated protein (PL-VAP), which is expressed in the endothelium of peritubular capillaries and vasa recta but not in glomeruli and arteries. Urinary PTC-EMP levels correlated directly with blood pressure and inversely with eGFR. In renovascular hypertensive subjects, urinary PTC-EMP levels correlated inversely with histological PTC count (r = −0.786, p=0.036) and directly with the percentage of fibrosis (r = 0.828, p=0.042).74 Levels of miR-29c, miR-21, E-cadherin mRNA, and vimentin mRNA in urinary exosomes were examined in 32 patients with IF/TA on renal biopsy and in 20 control patients with no IF/TA in renal biopsy; IF/TA was evaluated with semiquantitative score. Compared with controls, urinary exosomal miR-21 and vimentin were significantly increased in IF/TA patients (p<0.05), whereas miR-29c (p<0.05) and E-cadherin (p=0.0524) were decreased, with miR-29c appearing the most reliable of the four.75
Considering the labor-intensive techniques to isolate microparticles and exosomes, the potential of these types of samples as a source of diagnostic biomarkers does not seem promising.
Identification of specific pathologic kidney injury and evaluation of its progression, in most cases, still require a kidney biopsy, in conjunction with the estimation of eGFR and quantification of urine protein excretion. Several urine and blood compounds, reviewed here, have been proposed as potential non-invasive biomarkers of chronic IF and TA. Such a tool would be invaluable for the clinical care of patients with kidney disease. Although the progress has been significant, and the results very encouraging, current data suggest that further study is necessary before sufficient validation and regulatory approval of soluble biomarkers to be available for use in routine clinical settings for renal fibrosis.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors have contributed to this article as follows: SMB and AZR wrote and edited the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Serena M. Bagnasco, Department of Pathology, School of Medicine, The Johns Hopkins University, Baltimore, Maryland.
Avi Z. Rosenberg, Department of Pathology, School of Medicine, The Johns Hopkins University, Baltimore, Maryland
Literature Cited
- 1. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration and National Institutes of Health; 2016. [PubMed] [Google Scholar]
- 2. Leptak C, Menetski JP, Wagner JA, Aubrecht J, Brady L, Brumfield M, Chin WW, Hoffmann S, Kelloff G, Lavezzari G, Ranganathan R, Sauer J-M, Sistare FD, Zabka T, Wholley D. What evidence do we need for biomarker qualification? Sci Transl Med. 2017;9(417):eaal4599. [DOI] [PubMed] [Google Scholar]
- 3. Rockey DC, Bell PD, Hill JA. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–49. [DOI] [PubMed] [Google Scholar]
- 4. Schaefer L. Decoding fibrosis: Mechanisms and translational aspects. Matrix Biol. 2018;68–69:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Leaf IA, Duffield JS. What can target kidney fibrosis? Nephrol Dial Transplant. 2017;32(Suppl. 1):i89–97. [DOI] [PubMed] [Google Scholar]
- 6. Pakshir P, Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68–69:81–93. [DOI] [PubMed] [Google Scholar]
- 7. Flevaris P, Vaughan D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin Thromb Hemost. 2017;43(2):169–77. [DOI] [PubMed] [Google Scholar]
- 8. Boor P, Floege J. Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant. 2015;15(4):863–86. [DOI] [PubMed] [Google Scholar]
- 9. Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87(2):297–307. [DOI] [PubMed] [Google Scholar]
- 11. Lin S-L, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173(6):1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19(12):2282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol. 2019;30(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramann R, Machado F, Wu H, Kusaba T, Hoeft K, Schneider RK, Humphreys BD. Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight. 2018;3(9):e99561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Striker GE, Schainuck LI, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Hum Pathol. 1970;1:615–30. [DOI] [PubMed] [Google Scholar]
- 18. Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970;1:631–41. [DOI] [PubMed] [Google Scholar]
- 19. Bohle A, Mackensen-Haen S, von Giese H, Grund K, Wehrmann M, Batz C, Bogenschütz O, Schmitt H, Nagy J, Müller C, Müller M. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract. 1990;186:135–44. [DOI] [PubMed] [Google Scholar]
- 20. Yamanouchi M, Wada T, Hoshino J, Takaichi K, Ubara Y, Kinowaki K, Fujii T, Ohashi K, Mise K, Toyama T, Hara A, Shimizu M, Furuichi K, Wada T. Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: a biopsy-based cohort study. Nephrol Dial Transplant. Epub 2018. May 19. doi: 10.1093/ndt/gfy121. [DOI] [PubMed] [Google Scholar]
- 21. Cattran D, Coppo R, Cook H, Feehally J, Roberts I, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–45. [DOI] [PubMed] [Google Scholar]
- 22. Haas M, Verhave JC, Liu Z-H, Alpers CE, Barratt J, Becker JU, Cattran D, Cook HT, Coppo R, Feehally J, Pani A, Perkowska-Ptasinska A, Roberts IS, Soares MF, Trimarchi H, Wang S, Yuzawa Y, Zhang H, Troyanov S, Katafuchi R. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol. 2017;28:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin HA, III, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE. Prognostic factors in lupus nephritis: contribution of renal histologic data. Am J Med. 1983;75(3):382–91. [DOI] [PubMed] [Google Scholar]
- 24. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D’Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noël LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–96. [DOI] [PubMed] [Google Scholar]
- 25. Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. [DOI] [PubMed] [Google Scholar]
- 26. Mariani L, Martini S, Barisoni L, Canetta P, Troost JP, Hodgin J, Palmer M, Rosenberg AZ, Lemley KV, Chien HP, Zee J, Smith A, Appel GB, Trachtman H, Hewitt SM, Kretzler M, Bagnasco SM. Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transplant. 2017;33:310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farris AB, Alpers CE. What is the best way to measure renal fibrosis? a pathologist’s perspective. Kidney Int Suppl. 2014;4(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22(1):176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farris AB, Chan S, Climenhaga J, Adam B, Bellamy COC, Serón D, Colvin RB, Reeve J, Mengel M. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14(4):897–907. [DOI] [PubMed] [Google Scholar]
- 30. Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, Palmer M, Rosenberg A, Gasim A, Liensziewski C, Merlino L, Chien HP, Chang A, Meehan SM, Gaut J, Song P, Holzman L, Gibson D, Kretzler M, Gillespie BW, Hewitt SM. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol. 2016;29:671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strupler M, Hernest M, Fligny C, Martin J-L, Tharaux P-L, Schanne-Klein M-C. Second harmonic microscopy to quantify renal interstitial fibrosis and arterial remodeling. J Biomed Opt. 2008;13:054041. [DOI] [PubMed] [Google Scholar]
- 32. Vuillemin N, Mahou P, Débarre D, Gacoin T, Tharaux P-L, Schanne-Klein M-C, Supatto W, Beaurepaire E. Efficient second-harmonic imaging of collagen in histological slides using Bessel beam excitation. Sci Rep. 2016;6:29863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ranjit S, Dobrinskikh E, Montford J, Dvornikov A, Lehman A, Orlicky DJ, Nemenoff R, Gratton E, Levi M, Furgeson S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016;90(5):1123–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang B, Sun Y, Zhou J, Wu X, Chen S, Wu S, Liu H, Wang T, Ou X, Jia J, You H. Advanced septa size quantitation determines the evaluation of histological fibrosis outcome in chronic hepatitis B patients. Mod Pathol. 2018;31(10):1567–77. [DOI] [PubMed] [Google Scholar]
- 35. Ducourthial G, Leclerc P, Mansuryan T, Fabert M, Brevier J, Habert R, Braud F, Batrin R, Vever-Bizet C, Bourg-Heckly G, Thiberville L, Druilhe A, Kudlinski A, Louradour F. Development of a real-time flexible multiphoton microendoscope for label-free imaging in a live animal. Sci Rep. 2015;5:18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peride I, Rădulescu D, Niculae A, Ene V, Bratu OG, Checheriță IA. Value of ultrasound elastography in the diagnosis of native kidney fibrosis. Med Ultrason. 2016;18(3):362–9. [DOI] [PubMed] [Google Scholar]
- 37. Wang Z, Yang H, Suo C, Wei J, Tan R, Gu M. Application of ultrasound elastography for chronic allograft dysfunction in kidney transplantation. J Ultrasound Med. 2017;36(9):1759–69. [DOI] [PubMed] [Google Scholar]
- 38. Leung G, Kirpalani A, Szeto SG, Deeb M, Foltz W, Simmons CA, Yuen DA. Could MRI be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin J Am Soc Nephrol. 2017;12(6):1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morrell GR, Zhang JL, Lee VS. Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol. 2017;28(9):2564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Stillfried S, Apitzsch JC, Ehling J, Penzkofer T, Mahnken AH, Knüchel R, Floege J, Boor P. Contrast-enhanced CT imaging in patients with chronic kidney disease. Angiogenesis. 2016;19(4):525–35. [DOI] [PubMed] [Google Scholar]
- 41. Nogueira A, Pires MJ, Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo. 2017;31(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gewin L. The many talents of transforming growth factor-β in the kidney. Curr Opin Nephrol Hypertens. 2019;28:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parrish AR. Chapter two—matrix metalloproteinases in kidney disease: role in pathogenesis and potential as a therapeutic target. In: Khalil RA, editor. Progress in molecular biology and translational science, vol. 148: Cambridge, MA: Academic Press; 2017. pp. 31–65. [DOI] [PubMed] [Google Scholar]
- 44. Henger A, Kretzler M, Doran P, Bonrouhi M, Schmid H, Kiss E, Cohen CD, Madden S, Porubsky S, Gröne EF, Schlöndorff D, Nelson PJ, Gröne HJ. Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression. Kidney Int. 2004;65(3):904–17. [DOI] [PubMed] [Google Scholar]
- 45. Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7(316):316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hotchiss H, Chu T, Hancock W, Schroppel B, Kretzler M, Schmid H, Liu Y, Dikman S, Akalin E. Differential expression of profibrotic and growth factors in chronic allograft nephropathy. Transplantation. 2006;81:342–49. [DOI] [PubMed] [Google Scholar]
- 47. Modena BD, Kurian SM, Gaber LW, Waalen J, Su AI, Gelbart T, Mondala TS, Head SR, Papp S, Heilman R, Friedewald JJ, Flechner SM, Marsh CL, Sung RS, Shidban H, Chan L, Abecassis MM, Salomon DR. Gene expression in biopsies of acute rejection and interstitial fibrosis/tubular atrophy reveals highly shared mechanisms that correlate with worse long-term outcomes. Am J Transplant. 2016;16:1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cippà PE, Sun B, Liu J, Chen L, Naesens M, McMahon AP. Transcriptional trajectories of human kidney injury progression. JCI Insight. 2018;3(22):123151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O’Connell PJ, Zhang W, Menon MC, Yi Z, Schröppel B, Gallon L, Luan Y, Rosales IA, Ge Y, Losic B, Xi C, Woytovich C, Keung KL, Wei C, Greene I, Overbey J, Bagiella E, Najafian N, Samaniego M, Djamali A, Alexander SI, Nankivell BJ, Chapman JR, Smith RN, Colvin R, Murphy B. Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: a multicentre, prospective study. Lancet. 2016;388(10048):983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mansour SG, Puthumana J, Coca SG, Gentry M, Parikh CR. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: a systematic review. BMC Nephrol. 2017;18(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Greenberg JH, Kakajiwala A, Parikh CR, Furth S. Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol. 2018;33(6):925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, Tian J, Fu H, Hou FF, Liu Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol. 2017;28(2):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang H, Zheng C, Lu Y, Jiang Q, Yin R, Zhu P, Zhou M, Liu Z. Urinary fibrinogen as a predictor of progression of CKD. J Am Soc Nephrol. 2017;12(12):1922–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim HR, Nam BY, Kim DW, Kang MW, Han J-H, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo TH, Kang SW, Han DS, Han SH. Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis. 2013;61(6):899–909. [DOI] [PubMed] [Google Scholar]
- 55. Cho N-J, Han D-J, Lee J-H, Jang S-H, Kang JS, Gil H-W, Park S, Lee EY. Soluble klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS ONE. 2018;13(3):e0194617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olauson H, Mencke R, Hillebrands J-L, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017;100:19–35. [DOI] [PubMed] [Google Scholar]
- 57. Kuro-O M. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant. 2018;34(1):15–21. [DOI] [PubMed] [Google Scholar]
- 58. Wang G, Kwan BC-H, Lai FM-M, Chow K-M, Ng K-CJ, Luk CC, Li PK, Szeto CC. Urinary sediment mRNA level of extracellular matrix molecules in adult nephrotic syndrome. Clin Chim Acta. 2016;456:157–62. [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Lieske JC, Alexander MP, Jayachandran M, Denic A, Mathew J, Lerman LO, Kremers WK, Larson JJ, Rule AD. Tubulointerstitial fibrosis of living donor kidneys associates with urinary monocyte chemoattractant protein. Am J Nephrol. 2016;43(6):454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grandaliano G, Gesualdo L, Bartoli F, Ranieri E, Monno R, Leggio A, Paradies G, Caldarulo E, Infante B, Schena FP. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58(1):182–92. [DOI] [PubMed] [Google Scholar]
- 61. Nowak N, Skupien J, Smiles AM, Yamanouchi M, Niewczas MA, Galecki AT, Duffin KL, Breyer MD, Pullen N, Bonventre JV, Krolewski AS. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 2018;93(5):1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Azukaitis K, Ju W, Kirchner M, Nair V, Smith M, Fang Z, Thurn-Valsassina D, Bayazit A, Niemirska A, Canpolat N, Bulut IK, Yalcinkaya F, Paripovic D, Harambat J, Cakar N, Alpay H, Lugani F, Mencarelli F, Civilibal M, Erdogan H, Gellermann J, Vidal E, Tabel Y, Gimpel C, Ertan P, Yavascan O, Melk A, Querfeld U, Wühl E, Kretzler M, Schaefer F. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. Epub 2019. March 20. doi: 10.1016/j.kint.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 63. Chen C, Lu C, Qian Y, Li H, Tan Y, Cai L, Weng H. Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci Rep. 2017;7(1):17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schauerte C, Hübner A, Rong S, Wang S, Shushakova N, Mengel M, Dettling A, Bang C, Scherf K, Koelling M, Melk A, Haller H, Thum T, Lorenzen JM. Antagonism of profibrotic microRNA-21 improves outcome of murine chronic renal allograft dysfunction. Kidney Int. 2017;92(3):646–56. [DOI] [PubMed] [Google Scholar]
- 65. Nariman-Saleh-Fam Z, Bastami M, Ardalan M, Sharifi S, Hosseinian Khatib SM, Zununi Vahed S. Cell-free microRNA-148a is associated with renal allograft dysfunction: implication for biomarker discovery. J Cell Biochem. 2019;120:5737–46. [DOI] [PubMed] [Google Scholar]
- 66. Zununi Vahed S, Poursadegh Zonouzi A, Mahmoodpoor F, Samadi N, Ardalan M, Omidi Y. Circulating miR-150, miR-192, miR-200b, and miR-423-3p as non-invasive biomarkers of chronic allograft dysfunction. Arch Med Res. 2017;48(1):96–104. [DOI] [PubMed] [Google Scholar]
- 67. Maluf DG, Dumur CI, Suh JL, Scian MJ, King AL, Cathro H, Lee JK, Gehrau RC, Brayman KL, Gallon L, Mas VR. The urine microRNA profile may help monitor post-transplant renal graft function. Kidney Int. 2014;85(2):439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zununi Vahed S, Omidi Y, Ardalan M, Samadi N. Dysregulation of urinary miR-21 and miR-200b associated with interstitial fibrosis and tubular atrophy (IFTA) in renal transplant recipients. Clin Biochem. 2017;50(1):32–9. [DOI] [PubMed] [Google Scholar]
- 69. Gholaminejad A, Abdul Tehrani H, Gholami Fesharaki M. Identification of candidate microRNA biomarkers in renal fibrosis: a meta-analysis of profiling studies. Biomarkers. 2018;23(8):713–24. [DOI] [PubMed] [Google Scholar]
- 70. Assmann TS, Recamonde-Mendoza M, de Souza BM, Bauer AC, Crispim D. MicroRNAs and diabetic kidney disease: systematic review and bioinformatic analysis. Mol Cell Endocrinol. 2018;477:90–102. [DOI] [PubMed] [Google Scholar]
- 71. Liang S, Cai G-Y, Duan Z-Y, Liu S-w, Wu J, Lv Y, Hou K, Li ZX, Zhang XG, Chen XM. Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol. 2017;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PST. Chapter three—urine exosomes: an emerging trove of biomarkers. In: Makowski GS, editor. Advances in Clinical Chemistry, vol. 78 Amsterdam, The Netherlands: Elsevier; 2017. pp. 103–22. [DOI] [PubMed] [Google Scholar]
- 73. Lv L-L, Cao Y-H, Pan M-M, Liu H, Tang R-N, Ma K-L, Chen PS, Liu BC. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin Chim Acta. 2014;428:26–31. [DOI] [PubMed] [Google Scholar]
- 74. Sun IO, Santelli A, Abumoawad A, Eirin A, Ferguson CM, Woollard JR, Lerman A, Textor SC, Puranik AS, Lerman LO. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension. 2018;72(5):1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chun-yan L, Zi-yi Z, Tian-lin Y, Yi-li W, Bao L, Jiao L, Wei-Jun D. Liquid biopsy biomarkers of renal interstitial fibrosis based on urinary exosome. Exp Mol Pathol. 2018;105(2):223–8. [DOI] [PubMed] [Google Scholar]