Abstract
Background:
Gestational diabetes is the second common disorder in pregnancy period, which is detected in 24–28 weeks of gestational age through screening tests. Low-grade systematic inflammation is associated with an increased risk of type 2 diabetes. C–Reactive Protein (CRP), an acute phase protein produced by hepatocytes, may be associated with diabetes. This study aimed to investigate serum levels of CRP in women with Gestational Diabetes Mellitus (GDM) and impaired glucose tolerance test compared with control subjects.
Materials and Methods:
This observational longitudinal study was conducted on 176 pregnant women in Isfahan. After administration of a CRP test in these women in the first trimester, a screening test of Glucose Challenge Test (GCT) with 50-g oral glucose was conducted in 24–28 weeks of gestational age. Then, CRP levels and GCT were compared.
Results:
Serum CRP levels were not significantly correlated with positive GCT among the women. In GDM patients, there was not a significant correlation between CRP and BMI.
Conclusions:
There was no significant correlation between maternal serum CRP level and gestational diabetes. Maternal serum CRP level in the first trimester cannot predict Intolerance Glucose Test (IGT) in 26–30 weeks of gestational age.
Keywords: Body mass index, C - reactive protein, inflammation, pregnancy, screening test for gestational diabetes mellitus
Introduction
Anatomical, physiological, and biochemical adjustments with pregnancy are notable and numerous.[1] They may lead to a progressed pathological condition and result in problems and diseases for the pregnant women. One of these disorders is Gestational Diabetes Mellitus (GDM).
GDM is the most common medical complication during pregnancy.[1] It refers to glucose intolerance occurring or diagnosed firstly in pregnancy.[2] It drives 4%–5% of the pregnancies to complicated ones in US.[1,3,4] Hunt et al. reported prevalence of diabetes in low- and high-risk populations as 2% and 4.9%–12.8%, respectively.[3] Prevalence of this disease has been reported 2.5%–15% in different societies.[5]
In Iran, it has been reported between 1.3% and 11.9%.[6] Due to its serious complications for the fetus and the mothers, its early diagnosis to prevent diseases and prenatal disorders is of great importance.[7,8,9] Researchers have tried to detect some methods to achieve early diagnosis of GDM based on its pathogenesis. Evidences show that subclinical inflammation is involved in pathogenesis of insulin resistance.[10,11,12] Therefore, numerous studies have been conducted to investigate the association between markers as C-Reactive Protein (CRP), plasminogen (activator of inhibitor 1), and interleukin 6 as the most important mediators of acute phase inflammatory cytokine to predict diabetes type 2. The results of aforementioned studies suggest the probable role of inflammation factors in causing diabetes.[13,14,15]
The association between diabetes type two and inflammatory factors from cytokines such as interleukin type 6 [IL 6 and tumor necrosis factor (TNFα)] results in increased Gestational Infection (GI) and stimulation of acute phase inflammatory response.[16] Findings have shown the association between GDM and the increase in inflammatory factors and stress as well as oxidative such as prostaglandins type F (Prostaglandin F2 alpha). As CRP is one of the markers of acute phase inflammation, it may help detection of those women, predisposed to GDM. Therefore, this study aimed to define the association between CRP with a screening test for gestational diabetes in pregnant women referred to health centers in Isfahan.
Materials and Methods
This observational longitudinal study was conducted on 176 pregnant women referring to healthcare centers in Isfahan, Iran, after obtaining approval of ethics committee in Isfahan University of Medical Sciences and after talking with pregnant women, explaining the study and getting them satisfied in the period of March–April 2013–2014.
The number of participants was calculated to be 220, based on α = 0.05, power = 0.80, and standard error = 0.6. Two hundred pregnant women were selected through convenience sampling and informed written consent was obtained.
Inclusion criteria were no history of hypertension, no involvement in liver and renal diseases based on prenatal care profiles, no cigarette smoking, no alcohol or drugs abuse, and no involvement in a diagnosed systemic disease.[17,18] Exclusion criteria were occurrences of any midwifery or medical problems during study (bleeding, miscarriage, premature delivery, placental abruption, still birth, premature rupture of membranes, pre-eclampsia, eclampsia, and placenta Previa leading to termination of pregnancy), no active participation in prenatal care plan, taking medication (except for complements during pregnancy), and diagnosis of multiple pregnancy. To conduct sampling, after preparation of the list of all healthcare centers covered by health network number 1 (n = 30) and 2 (n = 28), some healthcare centers were randomly selected from these two clusters. After selection of the centers, with regard to the population, covered by each center, and calculated sample size, the subjects' number in each center was calculated. Finally, convenient sampling was conducted among the subjects meeting inclusion criteria to achieve the target subjects' number. Sampling continued during July 2013 to April 2014. It should be noted that in case of no women's further referral for prenatal care, it was followed up through phone calls.
After subject drop (4 due to miscarriage under 20 week of gestational age, 16 due to no referral for further prenatal care, and 24 subjects due to a change in national protocol and not undergoing GCT), the study was conducted with final subject number of 176 pregnant women.
After selection of qualified subjects and attaining an informed consent from them, mothers' demographic characteristics and history of previous diseases and pregnancies were recorded. During gestational age of 6–10 weeks, a 1 to 2-cc blood sample was taken to measure the level of CRP and was kept in −200°C after centrifuging and after maximum of 2 weeks, level of CRP was measured. All blood samples were sent to laboratory after being frozen with respect to cold chain. Mothers' height, weight, and BMI were measured and recorded. The next visit was conducted during 26–30 weeks of gestational age and GCT was requested for all the subjects. If blood sugar was equal or over 130 mg/dL 1 h after taking 50 g glucose, it was considered as a positive screening test result.
CRP monitoring test was administrated by enzyme immunoassay kit; IBL made in Canada and device of Stst FAX 303 through Eliza method in fluid and electrolyte research center of Isfahan University of Medical Sciences. Data were 19 entered in SPSS (Statistical Package for the Social Sciences IBM, Version 19). Mean, SD, and frequency distribution tables were presented. Independent t-test and Chi-square test were used to compare quantitative and qualitative variables, and linear regression test was adopted to define the association between CRP and GCT test results. p < 0.05 was considered as significance level for all statistical tests.
Ethical considerations
The study was approved by Medical Research Ethics Committee of Isfahan University of Medical sciences (392171) and was conducted in full compliance with the requirements of the Declaration of Helsinki regarding the use of human subjects.
Results
This study was conducted on 176 pregnant women referring to healthcare centers to receive prenatal care.
Mean (SD) age of the women, at 26–30 weeks of gestational age were 29.50 (4.39) and 26.80 (4.61) years in women with normal and positive GCT test results, respectively, with a significant difference (p = 0.001). About 38% of the subjects had education level less than high school and 94.90% were homemakers. Mean BMI of weeks 6–10 in women with normal and positive GCT results were 23.75 (3.75) and 25.21 (24.40), with a significant difference (p = 0.04). Mean pregnancy number and pregnancy rank in women with normal and positive GCT results were 1.80 (0.88) and 0.75 (0.75), and 2.11 (1.11) and 0.95 (0.90), respectively, Table 1.
Table 1.
Demographic characteristics of study sample
| Variable | Number (%) |
|---|---|
| Educational level | |
| Illiterate | 8 (4.50) |
| Under diploma | 59 (33.50) |
| Diploma and higher | 92 (52.30) |
| Bachelor’s degree and higher | 17 (9.70) |
| Occupational status | |
| Housewife | 167 (94.90) |
| Employed | 9 (5.10) |
| Delivery rank | |
| 0 | 68 (38.60) |
| 1 | 85 (48.30) |
| 2 | 18 (10.20) |
| 3 | 5 (2.80) |
| Pregnancy rank | |
| 1 | 64 (36.40) |
| 2 | 77 (43.8) |
| 3 | 27 (15.30) |
| 4 | 8 (4.50) |
Independent t-test showed a significant difference between two groups concerning pregnancy rank (p = 0.08) and delivery rank (p = 0.18). Based on the results, the maximum and minimum serum levels of CRP were 21.2 and 0.033, respectively. The results showed that 78.40% and 21.6% of the subjects had normal and positive screening test results. Linear regression test showed no significant correlation between CRP in weeks 6–10 and GCT in weeks 26–30 (β = 0.12, Figure 1). Regression test showed no correlation between serum CRP levels with GCT test at week 26–30 (r = 0.12).
Figure 1.
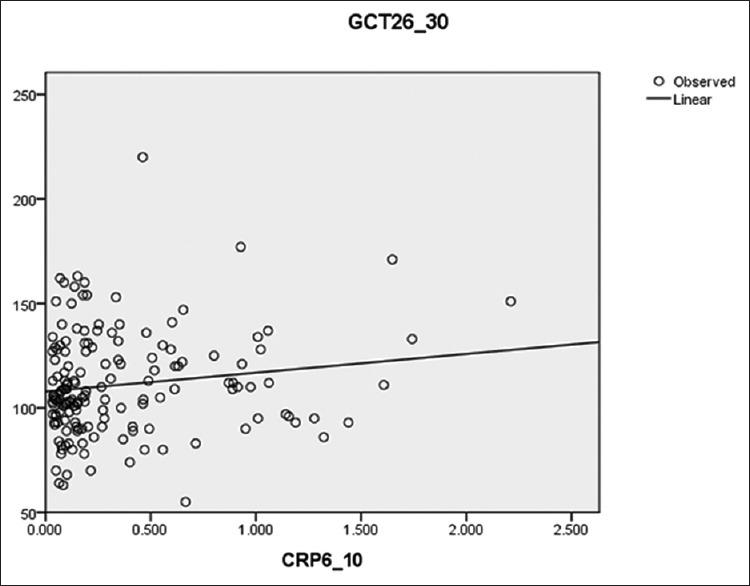
Determining the Relationship between serum CRP levels and GCT test results in pregnant women
Discussion
With regard to the obtained findings, pregnant women with increased level of CRP in the first trimester (weeks 6–10) are not at a higher risk of impaired glucose tolerance (IGT) or GDM in weeks 24–28. It can be concluded that CRP in the first trimester is not a valid predicting value for probability of IGT and GDM in the second half of pregnancy if BMI is the same in women with normal and positive GTT. This test cannot be counted as a reliable test to detect women with high risk of GDM. Women with normal BMI in the first trimester have no higher risk for future GDM. The increase in BMI, either in its normal or abnormal range, seems to play a role in significant increase of CRP serum level. Therefore, the increase in CRP with cutoff point of 130 mg/dL in weeks 6–10 of gestational age in women with normal BMI cannot predict the probability of IGT or GDM. Therefore, complementary CRP test, with regard to its high cost, cannot increase the capability of prediction of IGT or GDM in the first trimester in women with normal BMI. This study aimed to define the association between CRP and GDM result in low-risk pregnant women concerning the risk of being involved in IGT in Isfahan. Results showed that the women with positive GCT had a higher mean age. As other studies, conducted on women with GDM showed, women's age is one of the determinants for the risk of GDM,[18] and the women with positive GDM test result had a higher age, compared with those with normal GDM test result.[19,20,21,22] This study showed that women with positive GCT test result had high BMI, compared with those with a normal GCT. Mojibian et al. reported a higher BMI before pregnancy in women with GDM, compared with healthy pregnant women.[12] Li et al. also reported a significant difference in BMI in GDM screening test group, compared with healthy pregnant women (p > 0.05) Grewal (2012), Yachi (2011), and Torloni (2009) also showed that BMI was higher in women with IGT or GDM compared with healthy population.[23,24,25] The weight before pregnancy, weight gain during pregnancy, and nutritional factors such as intake of saturated fatty acids are among other risk factors associated to GDM.[26] The reason for the difference in results of other studies and this study is exclusion of pregnant women with BMI ≥30 kg/m2 in this study. Based on the findings, mean CRP serum level was higher in the group with positive GDM result, compared with normal screening test result. Previous studies reported that regardless of cutoff point of 130 or 140 mg/dL for diabetes screening test, there was a significant association between an increase in CRP serum level and positive GCT test and GDM.[15,16,27,28] Batachari et al. (2007) showed that mean CRP serum level in women with GDM was higher, compared to healthy women although the difference was not significant. In their study on obese women, they reported that CRP serum level in women with BMI >30 was significantly higher than those with BMI <30. They also reported that CRP is highly associated with obesity and seems to act as a factor to increase the risk of GDM.[23] Mojibian et al. (2011) showed that CRP serum level was not correlated with GDM in pregnant women, and CRP serum level in pregnant women could not predict the probability of women's IGT or GDM. On the other hand, in their study, they reported a significant correlation between CRP and before pregnancy BMI in pregnant women.[13] Based on our obtained results, there was no association between CRP at early months of pregnancy and positive GCT results in low-risk women. Therefore, the increase in CRP level in weeks 6–10 of pregnancy in women with normal BMI cannot predict IGT or GDM. Application of a complementary test such as CRP in women with normal BMI in the first trimester, with regard to its high cost, cannot increase the capability of prediction of IGT. In this regard, two points should be noted: First, most of the conducted studies investigated CRP serum level at the beginning of the second trimester or the third trimester, and then, with GCT and OGTT in weeks 26–30 of gestational age.[19,20,26,27,28] Second, after vast search of the researcher in available sites, only in one study, CRP level was used to predict the probability of IGT in the first trimester. In the limited existing conducted studies, all pregnant women (low and high risk), including overweight women with BMI >26 and obese with BMI over 30 referring to clinic for prenatal care, were entered to the study, whereas those with a diagnosed disease, history of GDM, endocrine disease (thyroid, hyperthyroidism, and adrenal), chronic hypertension, and inflammatory disease were not entered. Finally, 0.9% of the subjects with IGT belonged to low-risk group, which is a notable percentage and reveals the need for paying more attention to this group.[13,16,27]
This study had some limitations, including the small number of the participants, loss of the samples, and incomplete registration of the data in the files. So, it is recommended that further studies would be conducted with larger sample size.
Conclusion
Findings showed that CRP level in the first trimester is not a valid criterion to predict probability of IGT or GDM in the second trimester. Therefore, this test cannot be used as a reliable test to detect low risk GDM women.
Financial support and sponsorship
Isfahan University of Medical Sciences
Conflicts of interest
Nothing to declare.
Acknowledgements
Authors wish to express their sincere gratitude to the authorities of education and research departments and ethics committee of University, and we thank pregnant women helped us during our research and midwives of health centers. The study has been approved by the University (grant no: 392171).
References
- 1.Cuningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Westrom KD. Text Book of Williams's Obstetrics: Diabetes Mellitus. 25th ed. New York: Mc Graw Hill Company; 2018. pp. 1317–19. 1 and 2. [Google Scholar]
- 2.Georgiou HM, López SI, Rice GE. (November 2nd 2011). Novel screening approaches for the early detection of gestational diabetes mellitus. Gestational Diabetes, Miroslav Radenkovic, IntechOpen. [Last accessed on 2011 Nov 02]. DOI: 10.5772/21976. Available from: https://www.intechopen.com/books/gestational-diabetes/novel-screeningapproaches-for-the-early-detection-of-gestational-diabetes-mellitus .
- 3.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2017;34:173–99. doi: 10.1016/j.ogc.2007.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbs RS, Danforth DK, Karlan BY, Haney AF. Danforth Obstetrics and Gynecology. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2008. pp. 1317–19. [Google Scholar]
- 5.Ardakani M, Rashidi M. Gestational diabetes. Hormozgan Med Sci. 2007;1:1–12. [Google Scholar]
- 6.Meybodi F, Mahmodi M, Hassani M. Attitude of gestational diabetes in pregnant women to health centers in Kerman University of medical sciences. Razi Med. 2011;11:17–24. [Google Scholar]
- 7.Diabetes in Pregnancy. Management of diabetes and its complications from preconception to the postnatal period. 2018. [Last accessed on 2008]. Available from: http://www.rcog.org.uk . [PubMed]
- 8.Farasel R, James D, Steer P, Weiner C, Gonik B. High Risk Pregnancy. 4th ed. China: Elsevier Saunders publisher; 2011. [Google Scholar]
- 9.Myles W, Laura S, Karen H, Jeffrey L, Ravi T. First –trimester's C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26:819–24. doi: 10.2337/diacare.26.3.819. [DOI] [PubMed] [Google Scholar]
- 10.Pendergrass M, Fazioni E, Defronzo RA. Non-insulin dependent diabetes mellitus and gestational diabetes mellitus: Same disease, another name. Diabetes Rev. 1995;3:566–83. [Google Scholar]
- 11.Clark CMU, Qiu C, Amerman B, Porter B, Fireberg N, Aldasouqi S. Gestational diabetes: Should it be added to the syndrome of insulin resistance. Diabets Care. 1997;20:867–71. doi: 10.2337/diacare.20.5.867. [DOI] [PubMed] [Google Scholar]
- 12.Mojibian M, Soheilykhan S, Rahimi Saghand S, Rashidi M. Maternal serum C-reactive protein concentration in gestational diabetes. Iran J Diabetes Obesity. 2011;3:2. doi: 10.1016/j.tjog.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cushman M, Stemfer MJ, Tracy RP, Hannekens CH. Inflamation, aspirin and the risk of cardiovascular disease in apparently health men. Engel J Med. 1997;336:9973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 14.Paradhan AD, Manson JE, Rifai N, Buring J, Ridker PM. C-reactive protein interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 15.Freeman DJ, Norrie J, Caslake MJ, Fard I, Lowe G, Oreilly D, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland coronary prevention study. Diabets. 2002;51:1596–600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Lu X. Study correlation between C-reactive protein and gestational Diabetes mellitus. Obstet Gynecol. 2007;21:382–5. [Google Scholar]
- 17.Najafipour F, Aliasgharzadeh A, Bahrami A, Niafar M, Mobasseri M. Prevalency of diabetic nephropathy among type 2 diabetic patients. J Gorgan Uni Med Sci. 2009;11:62–6. [Google Scholar]
- 18.Garshasbi A, Zamirry A, Faghihzadeh S, Naghizadeh MM. Comprative evaluation of fasting plasma glucose and one hour 50-gr glucose challenge test in screening gestational diabetes mellitus. Zanjan Univ Med Sci J. 2010;18:1–12. [Google Scholar]
- 19.Yachi Y, Tanaka Y, Anasako Y, Nishibata I, Saito K, Sone H, et al. Contribution of first trimester fasting plasma insulin levels to the incidence of glucose intolerance in later pregnancy: Tanaka women s clinic study. Diabetes Res Clin Pract. 2011;92:293–8. doi: 10.1016/j.diabres.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Kashi Z, Borzouei SH, Akha O, Moslemzadeh N, Zakeri HD, Mohammad Poor A. Diagnostic value of fasting plasma glucose in screening of gestational diabetes mellitus. Int Endocrinology Metab. 2007;1:5–8. [Google Scholar]
- 21.Lopolla A, Dalfra MG, Mello G, Parretti E, Cioni R, Marzari C. Early detection of insulin sensitivity and B-cell function with simple test indicates future derangements in late pregnancy. J Clin Endocrinol Metab. 2008;93:876–80. doi: 10.1210/jc.2007-1363. [DOI] [PubMed] [Google Scholar]
- 22.Riskin-Mashiah S, Damit A, Younes G, Auslender R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur J Obster Gynecol Reprod Biol. 2010;152:163–7. doi: 10.1016/j.ejogrb.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 24.Grewal E, Kansara S, Kachhawa G, Ammini AC, Kriplani A, Aggarwal N, et al. Predict of gestanal diabetes mellitus at 24 to 28 weeks of gestational by using first-trimester insulin sensivity indices in Asian Indian subjects. Metabolism. 2012;61:715–20. doi: 10.1016/j.metabol.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Mirfeizi M, Mehdizadeh Tooeazani Z, Asghari Jafarabedi M, Gholami M, Moniri Tekmehdash A. Examining diagnostic value of the fasting plasma glucose in screening gestational Diabetes. Iran J Diabetes Lipid Dis. 2011;10:1–5. [Google Scholar]
- 26.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2003;21:103–13. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 27.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2011;9:414–7. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–7. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]


