Abstract
Background:
Venous thromboembolism (VTE) is a common and potentially lethal disorder that manifests mainly as deep vein thrombosis (DVT) of the extremities or pulmonary embolism (PE) and occurs as a consequence of genetic and environmental risk factors. We aimed to assess the role of inherited thrombophilia as a causative or additive factor in the development of VTE.
Methods:
The study included 310 patients (female: 154; mean age: 52.3 ± 16.9 years) with a first episode of VTE and 289 age- and sex-matched healthy controls. All participants underwent screening for thrombophilia-associated polymorphisms including factor V Leiden (FVL), prothrombin G20210A (PTG), factor V H1299 R (factor V HR2), factor XIII V34 L, β-fibrinogen-455 G>A, plasminogen activator inhibitor-1 4G/5G, human platelet antigen-1 a/b, methylene tetrahydrofolate reductase (MTHFR) C677 T, MTHFR A1298C, angiotensin-converting enzyme I/D, apolipoprotein B R3500Q, and apolipoprotein E (Apo E). In addition, serum homocysteine (Hcy) levels were measured.
Results:
In the patient group, 247 (80%) had isolated DVT, 43 (14%) had DVT plus PE, and 20 (6%) had isolated PE. The mean Hcy levels were similar in VTE subgroups and controls. Compared to controls, patients with isolated DVT, DVT plus PE, and isolated PE showed significantly higher frequencies for the following—heterozygous FVL mutation, isolated DVT (28.3%), DVT plus PE (44.2%), isolated PE (50%), controls (8.3%; P < .001); heterozygous PTG mutation, isolated DVT (11.3%), DVT plus PE (20.9%), isolated PE (25%), controls (5.9%; P < .01); Apo E 2/4, isolated DVT (9.7%), DVT plus PE (9.3%), isolated PE (5%), controls (1%; P < .01).The MTHFR A1298C mutation showed a significantly higher frequency in isolated patients with PE than in those with isolated DVT (P = .006) and in controls (P = .008). The frequencies of other genetic mutations or polymorphisms showed similar frequencies in all comparisons. In logistic regression analysis, heterozygous FVL mutation was the only independent predictor of VTE (odds ratio: 3.9, 95% confidence interval: 1.3-11.2; P = .012).
Conclusion:
Except than FVL, PTG, and Apo E 2/4 mutations, many of aforementioned thrombophilic factors known to be associated with VTE did not demonstrate any relationship with VTE. Heterozygous mutation of FVL was an independent predictor for VTE.
Keywords: thromboembolism, thrombophilia, pulmonary embolism, venous thrombosis
Introduction
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are considered to be diverse manifestations of the same disease termed venous thromboembolism (VTE). It is the third most frequent cardiovascular disease. One-third of VTE-related deaths resulted from sudden fatal PE, and undiagnosed PE was found to be the cause of VTE-related deaths in 59% of cases.1 Although available data about the prevalence of inherited thrombophilia in patients with VTE are controversial, it is detectable in at least 30% to 40% of these patients.1,2
Factor V Leiden (FVL) is the most common and well-studied genetic cause of VTE, followed by the prothrombin G20210A (PTG) gene mutation and deficiencies in protein S, protein C, and antithrombin. Some other mutations and polymorphisms have also been proposed as genetically determined procoagulant risk factors for VTE.1–5
In this single-center study aiming to compare a large series of patients with VTE to healthy controls, we examined 12 mutations or polymorphisms and serum homocysteine (Hcy) levels, which were previously reported to be associated with thrombophilia and diverse presentations of VTE.
Methods
This case–control tertiary single-center study included 310 patients (female = 154; age: 52.3 ± 16.9 years) with a first episode of VTE (DVT and/or PE) and 289 sex- and age-matched healthy controls (female = 126; age: 49.7 ± 14.7 years) without any cardiovascular disease or VTE events. Based on history, VTE is considered to be “provoked” or “unprovoked” in the presence or absence of a temporary or reversible predisposing risk factor (eg, surgery, trauma, immobilization, pregnancy, oral contraceptive use, or hormone replacement therapy) within the last 6 weeks to 3 months before diagnosis, respectively.1 The diagnosis of DVT was based on the findings of compression ultrasonography.6,7 Diagnosis of definite PE was objectively confirmed by 64-slice multidetector computed tomographic pulmonary angiography according to the current guidelines for PE.1 All patients with PE at presentation underwent an ultrasonographic study of the lower limbs and abdomen for DVT within 7 days. All patients were screened for the presence of any occult malignancy. Screening data were obtained after 6 months to 1 year following the acute VTE episode, at least 1 month after discontinuation of vitamin K antagonists, provided that there had been no severe inflammatory disorders for at least 6 months.
Screening for Thrombophilia
The study protocol was approved by the Institutional Ethics Committee, and a written informed consent was obtained from all patients and controls. All patients and controls underwent laboratory screening for thrombophilia-associated polymorphisms including FVL, PTG, factor V H1299 R (factor V HR2), factor XIII V34 L, β-fibrinogen-455 G>A, plasminogen activator inhibitor-1 (PAI-1) 4G/5G, human platelet antigen-1 a/b, methylene tetrahydrofolate reductase (MTHFR) C677 T, MTHFR A1298C, angiotensin-converting enzyme (ACE) I/D, apolipoprotein B R3500Q (Apo B), and apolipoprotein E (Apo E). In addition, fasting serum Hcy levels were measured. Laboratory screening and Hcy measurements were performed according to the methods previously reported (Table 1).8,9
Table 1.
Thrombophilic Parameters Those Studied.
| Factor V Leiden |
| Prothrombin G20210A |
| Factor V H1299 R |
| Factor XIII V34L |
| β-fibrinogen-455 G>A |
| Plasminogen activator inhibitor-1 4G/5G |
| Human platelet antigen-1 a/b |
| Methylene tetrahydrofolate reductase C677T |
| Methylene tetrahydrofolate reductase A1298C |
| Angiotensin-converting enzyme I/D |
| Apolipoprotein B R3500Q |
| Apolipoprotein E polymorphisms |
| Homocysteine |
Statistical Analysis
Data were processed using the SPSS version 16.0 package (Statistical Package for Social Sciences Inc, Chicago, Illinois) and were expressed as number, mean, standard deviation, minimum and maximum for continuous variables, and number and percent for categorical variables, respectively. When continuous variables did not show a normal distribution, the Mann-Whitney U test was used. Categorical variables were analyzed using the Pearson χ2 and Fisher tests. A logistic regression analysis model was used to describe the association between VTE and thrombophilic risk factors. Odds ratios (ORs) were given with their 95% confidence intervals (CIs). All probability values were 2-tailed, and a P value of less than .05 was considered statistically significant.
Results
The clinical characteristics of the patients are summarized in Table 2, with anatomical locations of venous thrombi. The patient and control groups were similar in terms of age and gender (P > .05).
Table 2.
The Clinical Characteristics of Patients With Venous Thromboembolism.
| Sex, n (%) | |
|---|---|
| Female | 154 (49.7) |
| Male | 156 (50.3) |
| Age, mean ± SD, years | 52.4 ± 16.9 |
| Distal DVT | 101 (32.6) |
| Proximal (iliofemoropopliteal) DVT | 78 (25.2) |
| Distal + proximal DVT | 112 (36.1) |
| Vena cava inferior thrombosis | 8 (2.6) |
| Upper extremity DVT | 7 (2.3) |
| Upper + lower extremity DVT | 4 (1.3) |
| Pulmonary embolism | 55 (17.7) |
| Connective tissue disorders | 6 (1.9) |
| Malignancy | 13 (4.2) |
| Pregnancy, oral contraceptive use | 19 (6.1) |
| Postoperative immobilization | 61 (19.7) |
| Iatrogenic (eg, IV catheter or cast) | 3 (1.0) |
Abbreviations: DVT, deep venous thrombosis; IV, intravenous; SD, standard deviation.
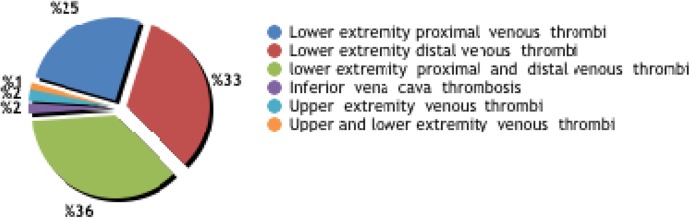
Of 310 patients, 247 (80%) had isolated DVT, 43 (14%) had DVT plus PE, and 20 (6%) had isolated PE (Figure 1). Isolated proximal thrombosis (in any iliac, femoral, or popliteal vein or in all 3 veins), isolated distal thrombosis (below the popliteal vein), and both proximal and distal thrombosis were seen in 78 (25.2%), 101 (32.6%), and 112 (36%) patients, respectively. Thrombosis was detected in the inferior vena cava in 8 (2.6%) patients. Deep vein thrombosis occurred in the upper extremity (in the axillary or subclavian vein) in 7 (2.3%) patients and in both lower and upper extremities in 4 (1.3%) patients.
Figure 1.
Anatomical locations of venous thrombi.
The frequencies of mutations or polymorphisms and Hcy levels in the VTE subgroups and controls are demonstrated in Table 3. The mean Hcy levels were similar in VTE subgroups and controls (16.1 ± 9.1 vs 15.6 ± 5.4 mol/L; P = .36). The Hcy levels also showed a similar distribution in patients with isolated DVT, DVT plus PE, and isolated PE (P = NS).
Table 3.
Gene Mutations or Polymorphisms and Homocysteine Levels in Patients With VTE and Controls.a
| Controls (n, %) (n = 289) | DVT + PE (n, %) (n = 43) | DVT (n, %) (n = 247) | PE (n, %) (n = 20) | P | |
|---|---|---|---|---|---|
| Homocysteine, mean ± SD, mol/L | 15.6 ± 5.4 | 16.1 ± 5.2 | 16.0 ± 9.5 | 15 ± 5.2 | .984 |
| Factor V G1691A | |||||
| Htr | 24 (8.3) | 19 (44.2) | 70 (28.3) | 10 (50.0) | <.001b |
| Hm | 1 (0.3) | 0 (0.0) | 17 (6.9) | 0 (0.0) | |
| Factor V H1299R | |||||
| Htr | 23 (8.0) | 9 (20.9) | 26 (10.5) | 2 (10.0) | .300 |
| Hm | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0) | |
| Prothrombin G 20210 | |||||
| Htr | 17 (5.9) | 9 (20.9) | 28 (11.3) | 5 (25.0) | .014b |
| Hm | 0 (0) | 0 (0.0) | 1 (0.4) | 0 (0) | |
| Factor 13 V34L | |||||
| Htr | 67 (23.2) | 15 (34.9) | 75 (30.4) | 3 (15.0) | .168 |
| Hm | 3 (1.0) | 2 (4.7) | 6 (2.4) | 1 (5.0) | |
| β-fibrinogen-455 | |||||
| Htr | 102 (35.3) | 15 (34.9) | 78 (31.6) | 6 (30.0) | .781 |
| Hm | 8 (2.8) | 3 (7.0) | 11 (4.5) | 1 (5.0) | |
| MTHFR C677T | |||||
| Htr | 129 (44.6) | 16 (37.2) | 104 (42.1) | 8 (40.0) | .591 |
| Hm | 31 (10.7) | 3 (7.0) | 33 (13.4) | 1 (5.0) | |
| MTHFR A1298C | |||||
| Htr | 123 (42.6) | 15 (34.9) | 24 (9.7) | 12 (60.0) | .005c |
| Hm | 19 (6.6) | 30 (69.8) | 4 (1.6) | 4 (20.0) | |
| PAI-1 | |||||
| 4G/4G | 81 (28.0) | 10 (23.3) | 59 (23.9) | 5 (25.0) | .735 |
| 4G/5G | 137 (47.4) | 19 (44.2) | 130 (52.6) | 11 (55.0) | |
| 5G/5G | 69 (23.9) | 14 (32.6) | 58 (23.5) | 4 (20.0) | |
| HPA-1(GP3a L33P) | |||||
| a/a | 228 (78.9) | 32 (74.4) | 191 (77.3) | 13 (65.0) | .621 |
| a/b | 52 (18.0) | 11 (25.6) | 50 (20.2) | 6 (30.0) | |
| b/b | 7 (2.4) | 0 (0) | 6 (2.4) | 1 (5.0) | |
| ACE | |||||
| D/D | 101 (34.9) | 17 (39.5) | 94 (38.1) | 13 (65.0) | .241 |
| I/D | 137 (47.4) | 18 (41.9) | 118 (47.8) | 5 (25.0) | |
| I/I | 49 (17.0) | 8 (18.6) | 33 (13.4) | 2 (10.0) | |
| Apo B | |||||
| Htr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
| Hm | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Apolipoprotein E | |||||
| E2/2 | 2 (0.7) | 0 (0.0) | 1 (0.4) | 0 (0) | |
| E2/3 | 28 (9.7) | 4 (9.3) | 25 (10.1) | 2 (10.0) | |
| E2/4 | 3 (1.0) | 4 (9.3) | 24 (9.7) | 1 (5.0) | .008b |
| E3/3 | 221 (76.5) | 29 (67.4) | 170 (68.8) | 14 (70.0) | |
| E3/4 | 31 (10.7) | 6 (14.0) | 27 (10.9) | 3 (15.0) | |
| E4/4 | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0) | |
Abbreviations: ACE, angiotensin-converting enzyme; Apo B, apolipoprotein B; DVT, deep venous thrombosis; hm, homozygous mutation; HPA-1, human platelet antigen-1; htr, heterozygous mutation; MTHFR, methylene tetrahydrofolate reductase; PAI-1, plasminogen activator inhibitor-1; PE, pulmonary embolism; VTE, venous thromboembolism.
aData are presented as mean ± standard deviation or number (%), where appropriate.
bPearson χ2 (Monte Carlo), P = .005; Fisher exact, P = .003. cFisher exact.
Heterozygous FVL mutations were significantly more frequent in patients with isolated DVT (28.3%), DVT plus PE (44.2%), and isolated PE (50%) as compared to controls (8.3%; P < .001). The incidence of heterozygous FVL mutations was higher in patients with distal plus proximal DVT than in those with proximal DVT (41 [41.3%] vs 15 [26.4%]; P = .01). Pulmonary embolism patients with or without DVT showed no homozygous FVL mutations. The frequency of the factor V HR2 haplotype, which is a set of 6 closely linked polymorphisms within the factor V gene, did not differ significantly in all comparisons (P = NS; Figure 2).
Figure 2.
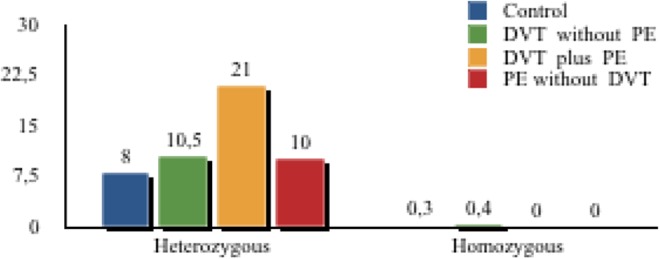
Frequency of factor V H1299R in controls and subgroups of venous thromboembolism (VTE).
The frequencies of heterozygous PTG mutations were significantly higher in patients with isolated DVT (11.3%), DVT plus PE (20.9%), and isolated PE (25%) as compared to controls (5.9%; P < .01). The frequencies of heterozygous PTG mutations were similar in the VTE subgroups (P = NS). Patients with VTE had a significantly higher frequency of double heterozygosity for FVL and PTG mutations as compared to controls (4.2% vs 0.3%; P = .0001).
Compared to controls (1%), patients with VTE also showed significantly higher rates for Apo E 2/4 mutation, with 9.7% for isolated DVT, 9.3% for DVT plus PE, and 5% for isolated PE (P = 008).
The MTHFR A1298C mutation (thermoresistant variant) showed a significantly higher frequency in isolated patients with PE than in those with isolated DVT (P = .006) and in controls (P = .008). All the remaining genetic parameters showed similar frequencies in all comparisons (Table 3).
In logistic regression analysis, homozygous PTG, Apo E2/2, and Apo E4/4 were excluded because of the small numbers of cases. Multivariate analysis showed that heterozygous FVL mutation was the only independent predictor of VTE (OR = 3.9; 95% CI: 1.3-11.2; P = .012; Table 4).
Table 4.
Univariate and Multivariate Analysis for Patients With Venous Thromboembolism.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| P | OR | Lower Limit | Upper Limit | P | OR | Lower Limit | Upper Limit | |
| Factor V G1691A | <.001 | .04 | ||||||
| Heterozygous | <.001 | 4.8 | 3 | 7.8 | .012 | 3.8 | 1.3 | 11.1 |
| Homozygous | .003 | 22.1 | 2.92 | 167.5 | .9 | 8.9 × 108 | <0.001 | – |
| Prothrombin G 20210a | .01 | 2.2 | 1.2 | 4 | .9 | 1 | 0.3 | 3.8 |
| Factor 13 V34L | .053 | .3 | ||||||
| Heterozygous | .04 | 1.45 | 1.01 | 2.099 | .1 | 2.9 | 0.8 | 5.3 |
| Homozygous | .133 | 2.79 | 0.73 | 10.67 | .9 | 0.8 | 0.06 | 11 |
| MTHFR A1298C | .065 | .5 | ||||||
| Heterozygous | .94 | 0.99 | 0.7 | 1.4 | .9 | 1.5 | 0.5 | 2.4 |
| Homozygous | .026 | 1.97 | 1.087 | 3.58 | .28 | 2.6 | 0.5 | 14.5 |
| Apolipoprotein | ||||||||
| E2/3 | .002 | .2 | ||||||
| E2/4 | .001 | 8.73 | 2.39 | 31.84 | .998 | 1.2 × 108 | <0.001 | – |
| E3/3 | .595 | 0.863 | 0.501 | 1.487 | .19 | 0.3 | 0.07 | 1.7 |
| E3/4 | .89 | 1.05 | 0.52 | 2.11 | .92 | 1.1 | 0.1 | 8 |
Abbreviations: CI, confidence interval; MTHFR, methylene tetrahydrofolate reductase; OR, odds ratio.
aAbsence versus presence of heterozygous mutation of prothrombin G 20210A was studied.
Discussion
Our single-center study is the first in which 12 genetic mutations or polymorphisms and serum Hcy levels were evaluated in a large series of patients with diverse clinical presentations of VTE and in healthy controls. Age and gender did not show any significant association with VTE. In our case–control analysis, we found that many of the thrombophilic factors that are likely to be associated with VTE did not demonstrate any relationship with VTE. Heterozygous FVL, PTG, and Apo E2/4 mutations were significantly associated with VTE. Moreover, MTHFR A1298C (thermostable variant) was significantly more frequent in isolated patients with PE as compared to those with isolated DVT and controls. Heterozygous FVL mutation was found to be an independent predictor of VTE regardless of the presentation. Patients with VTE have some components of genetically determined thrombophilia that are associated with loss-of-function of anticoagulant proteins or gain-of-function of procoagulants.1
Factor V Leiden is the most frequent and well-studied genetic cause of VTE, followed by the PTG mutation.10–13 The prevalence of FVL carriage is 5% among Caucasians. This condition was documented in 20% of patients with DVT and approximately 50% of patients with familial thrombophilia. For FVL mutation, the risk of thrombosis is reported to increase 5-fold in heterozygotes and 50-fold in homozygotes. This common mutation is considered to be responsible for 20% to 25% of VTE events, with higher recurrence rates of DVT during an 8-year follow-up period.
The PTG mutation leads to an increased prothrombin production, which may amount to 30% to 70% higher levels of prothrombin in heterozygotes and homozygotes as compared to the absence of this condition. Therefore, PTG mutation is associated with nearly 3 times the higher risk of VTE. This mutation was found in 2% to 3% of western population but was reported to be rare in other regions.14,15
Moreover, synergism appears to exist between PTG mutation and delayed activation of FVL, a substrate, and cofactor in the prothrombinase complex.15–17 In a pooled analysis of 8 case–control studies comprising large numbers of patients and controls, the OR for VTE was 4.9 (95% CI: 4.1-5.9) with FVL mutation and 3.8 (95% CI: 3.0-4.9) with PTG mutation. Our study demonstrated that patients with VTE had a significantly higher frequency of double heterozygosity for FVL and PTG mutations, although double heterozygosity was unrelated with specific presentations of VTE.
Unlike FVL mutation that is more prevalent in Caucasian populations, the factor V HR2 haplotype was found at similar frequencies among individuals of diverse races.18 When accompanied by FVL mutation, this condition was associated with an additional 3- to 4-fold increase in DVT risk compared with that associated with FVL mutation alone.18–20
Factor XIII has a transglutaminase activity and plays an essential role in fibrin cross-linking, resulting in an increased resistance against premature fibrinolysis.21 The factor XIII Val34Leu polymorphism was reported to be associated with an increased VTE risk in some studies, but this association was not verified by other studies.22–25
Individuals with MTHFR variants may have slight elevations in Hcy levels, especially when folate deficiency is present.26 The most common MTHFR allele is the C677 T (thermolabile) variant. The second most common allele, A1298C, is seen in the heterozygous state in nearly 9% to 20% of diverse ethnic groups.27
Mild to moderate elevations in Hcy levels have been reported to be associated with VTE in young individuals and with recurrent DVT.28,29 A meta-analysis of 53 studies on the MTHFR 677TT genotype, which increases Hcy, suggested a 20% higher risk of DVT (95% CI: 8-32) compared to that associated with the MTHFR 677CC genotype. In contrast to the findings of non-American studies, the MTHFR genotypes were found to have no effect on DVT in North America due probably to the higher intake of folate and riboflavin in this population.29,30
The β-chain synthesis has been shown to be a rate-limiting step in fibrinogen production, and any mutation related with the β-fibrinogen gene or its transcription may have an impact on plasma fibrinogen levels.31 However, the association between the β-fibrinogen gene polymorphism and VTE remains controversial.32
The overexpression of PAI-1 leads to an impairment in the activity of the fibrinolytic system, thereby increasing the risk for thrombotic events.33 The gene for PAI-1 has several polymorphic loci, including a 4G/5G sequence polymorphism 675 base pairs from the start of the promoter, with the 4G polymorphism resulting in 25% higher levels of transcription.34 A meta-analysis showed a significant relationship between the PAI-1 4G polymorphism and an increased risk for VTE.35
A polymorphism (insertion/deletion; I/D allele) in the ACE gene was found to account for approximately one-third to one-half the variance in plasma ACE levels.36 Some interactions seem to exist between the renin–angiotensin system and fibrinolysis, and the D allele may have an association with higher levels of PAI-1 and a certain degree of hypofibrinolysis.37 However, the relationship between the ACE I/D polymorphism and VTE risk was shown only in African American males, whereas this association remains controversial in general population or among thrombophilic patients of Caucasian origin.38,39
Apolipoprotein E is a plasma glycoprotein that determines the variation in plasma cholesterol levels. In comparison to the carriers of the E3/E3 allele, carriers of the E2 allele tend to have increased levels of Apo E and decreased levels of low-density lipoprotein cholesterol, whereas E4 carriers tend to have decreased levels of Apo E and increased levels of high-density lipoprotein cholesterol.40 High plasma levels of Apo B, which results in the subendothelial retention of lipoprotein-containing Apo B, is also an important step in atherogenesis.41
All patients with VTE, whether they have a known inherited thrombophilia, are prone to recurrent thromboses for many years after the index event. Diagnosis of genetic susceptibility to thrombophilia rarely affects the acute or long-term management of VTE, whereas the risk of recurrent VTE is increased in anticoagulant-deficient patients and in homozygotes for gain-of-function mutations.1 Recurrence is fatal in approximately 5% of patients, and in one-third of patients, it is associated with postthrombotic syndrome.1,42 According to the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) 2014 PE guidelines, among carriers of molecular thrombophilia, patients with a lupus anticoagulant, those with a confirmed deficiency in protein C or protein S, and patients with a homozygous FVL or homozygous PTG mutation may be candidates for indefinite anticoagulant treatment after a first unprovoked VTE.1 Notably, the term ‘indefinite anticoagulation’ is not synonymous with ‘lifelong treatment’; it simply indicates that the duration of treatment cannot be defined at three-month follow-up after the acute event. 1 Because of the absence of evidence for the clinical benefit of extended anticoagulant treatment for carriers of heterozygous FVL or PTG mutations, a periodical reassessment is recommended in these patients for option to withdraw anticoagulant treatment based on the dynamic balance between the risks of recurrence and bleeding.1 Lifelong treatment is recommended for most patients with a second unprovoked DVT or PE.1
Study Limitations
Even though our results were derived from a case–control analysis including a large series of patients with diverse presentations of VTE and an acceptable number of healthy controls, further studies with larger patient series and multicenter design are needed to address the individual and synergistic relationships between inherited thrombophilic mutations or polymorphisms and VTE risk. The lack of comparisons between unprovoked and provoked VTE in terms of genetic susceptibility to thrombotic risk is another important limitation of this study. Moreover, our study does not propose a specific predictor for the occurrence of PE at presentation with DVT or during mid- to long-term follow-up period of anticoagulant treatment. Debate seems to continue over the impact of genetically determined thrombophilia on risk stratification and anticoagulant management planning in this setting.
Conclusion
Our study comprising a large series of patients with VTE showed that the frequencies of FVL, PTG, and Apo E 2/4 mutations were significantly higher in patients with VTE. Other potentially thrombophilic genetic mutations or polymorphisms and Hcy levels were not related with VTE risk. Heterozygous FVL mutation was the only independent predictor of VTE. Further studies are needed to assess the importance of genetically determined thrombophilia for the risk stratification of patients with VTE and planning the duration of anticoagulant treatment.
Footnotes
Authors’ Note: Oral presentation (with the title of Genetic determinants in venous thrombosis) at the European Society of Cardiology Congress 2008, Munich, Germany, August 30 to September 3, 2008. This preliminary report was published as an abstract with 192 (now 310) patients and 118 (now 289) controls in the Eur Heart J (2008) 29 (suppl 1).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. [DOI] [PubMed] [Google Scholar]
- 2. Cushman M. Inherited risk factors for venous thrombosis. Hematol Am Soc Hematol Educ Program. 2005:452–457. [DOI] [PubMed] [Google Scholar]
- 3. Trégouët DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113(21):5298–5303. [DOI] [PubMed] [Google Scholar]
- 4. D Klai S, Fekih-Mrissa N, Sassi RB, Mrissa R, Rachdi R, Gritli N. Effects of prothrombotic markers and non-O blood group in maternal venous thromboembolism during pregnancy and postpartum. Blood Coagul Fibrinolysis. 2012;23(7):649–652. [DOI] [PubMed] [Google Scholar]
- 5. Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38(5):535–548. [DOI] [PubMed] [Google Scholar]
- 6. Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med. 1998;129(12):1044–1049. [DOI] [PubMed] [Google Scholar]
- 7. Perrier A, Bounameaux H. Ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1998;128(3):243; author reply 244-245. [DOI] [PubMed] [Google Scholar]
- 8. Güler GB, Batgerel U, Güler E, et al. Factor XIII Val34Leu polymorphism in patients with cardiac syndrome X. Cardiol J. 2014;21(1):6–10. [DOI] [PubMed] [Google Scholar]
- 9. Grifoni E, Marcucci R, Ciuti G, et al. The thrombophilic pattern of different clinical manifestations of venous thromboembolism: a survey of 443 cases of venous thromboembolism. Semin Thromb Hemost. 2012;38(2):230–234. [DOI] [PubMed] [Google Scholar]
- 10. Limperger V, Klostermeier UC, Kenet G, et al. Clinical and laboratory characteristics of children with venous thromboembolism and protein C-deficiency: an observational Israeli-German cohort study. Br J Haematol. 2014;167(3):385–393. [DOI] [PubMed] [Google Scholar]
- 11. Wu C, Dwivedi DJ, Pepler L, et al. Targeted gene sequencing identifies variants in the protein C and endothelial protein C receptor genes in patients with unprovoked venous thromboembolism. Arterioscler Thromb Vasc Biol. 2013;33(11):2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirmerova J, Seidlerova J, Subrt I. The association of factor V Leiden with various clinical patterns of venous thromboembolism—the factor V Leiden paradox. QJM. 2014;107(9):715–720. [DOI] [PubMed] [Google Scholar]
- 13. Limperger V, Franke A, Kenet G, et al. Clinical and laboratory characteristics of paediatric and adolescent index cases with venous thromboembolism and antithrombin deficiency. An observational multicentre cohort study. Thromb Haemost. 2014;112(3):478–485. [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM, Hennekens CH, Miletich JP. G20210A mutation in prothrombin gene and risk of myocardial infarction, stroke, and venous thrombosis in a large cohort of US men. Circulation. 1999;99(8):999–1004. [DOI] [PubMed] [Google Scholar]
- 15. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369–384. [DOI] [PubMed] [Google Scholar]
- 16. Koeleman BP, Reitsma PH, Allaart CF, Bertina RM. Activated protein C resistance as an additional risk factor for thrombosis in protein C-deficient families. Blood. 1994;84(4):1031–1035. [PubMed] [Google Scholar]
- 17. Zoller B, Garcia DF, Hillarp A, Dahlbäck B. Thrombophilia as a multigenic disease. Haematologica. 1999;84(1):59–70. [PubMed] [Google Scholar]
- 18. Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood. 1997;90(4):1552–1557. [PubMed] [Google Scholar]
- 19. de Visser MC, Guasch JF, Kamphuisen PW, Vos HL, Rosendaal FR, Bertina RM. The HR2 haplotype of factor V: effects on factor V levels, normalized activated protein C sensitivity ratios and the risk of venous thrombosis. Thromb Haemost. 2000;83(4):577–582. [PubMed] [Google Scholar]
- 20. Castaman G, Faioni EM, Tosetto A, Bernardi F. The factor V HR2 haplotype and the risk of venous thrombosis: a meta-analysis. Haematologica. 2003;88(10):1182–1189. [PubMed] [Google Scholar]
- 21. Kholer HP, Stickland MH, Ossei-Gerning N, Carter A, Mikkola H, Grant PJ. Association of a common polymorphism in the factor XIII gene with myocardial infarction. Thromb Haemost. 1998;79(1):8–13. [PubMed] [Google Scholar]
- 22. Cushman M, Cornell A, Folsom AR, et al. Associations of the β-fibrinogen Hae III and factor XIII Val34Leu gene variants with venous thrombosis. Thromb Res. 2007;121(3):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balogh I, Szôke G, Karpati L, et al. Val34Leu polymorphism of plasma factor XIII: biochemistry and epidemiology in familial thrombophilia. Blood. 2000;96(7):2479–2486. [PubMed] [Google Scholar]
- 24. Corral J, Gonzalez-Conejero R, Iniesta JA, Rivera J, Martínez C, Vicente V. The FXIII Val34Leu polymorphism in venous and arterial thromboembolism. Haematologica. 2000;85(3):293–297. [PubMed] [Google Scholar]
- 25. Margaglione M, Bossone A, Brancaccio V, Ciampa A, Di Minno G. Factor XIII Val34Leu polymorphism and risk of deep vein thrombosis. Thromb Haemost. 2000;84(6):1118–1119. [PubMed] [Google Scholar]
- 26. Pereira AC, Schettert IT, Morandini Filho AA, Guerra-Shinohara EM, Krieger JE. Methylenetetrahydrofolate reductase (MTHFR) c677t gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin Chim Acta. 2004;340(1-2):99–105. [DOI] [PubMed] [Google Scholar]
- 27. Arruda VR, von Zuben PM, Chiaparini LC, Annichino-Bizzacchi JM, Costa FF. The mutation Ala677–> Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Haemost. 1997;77(5):818–821. [PubMed] [Google Scholar]
- 28. den Heijer M, Koster T, Blom HJ, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334(12):759–762. [DOI] [PubMed] [Google Scholar]
- 29. Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3(2):292–299. [DOI] [PubMed] [Google Scholar]
- 30. Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Homocysteine: an emerging cardiovascular risk factor that never really made it. Open Clin Chem J. 2010;3:19–24. [Google Scholar]
- 31. Roy SN, Mukhopadhyay G, Redman CM. Regulation of fibrinogen assembly. Transfection of Hep G2 cells with Bb cDNA specifically enhances synthesis of the three component chains of fibrinogen. J Biol Chem. 1990;265(11):6389–6393. [PubMed] [Google Scholar]
- 32. Koster T, Rosendaal FR, Reitsma PH, et al. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA Polymorphisms—the Leiden Thrombophilia Study (LETS). Thromb Haemost. 1994;71(6):719–722. [PubMed] [Google Scholar]
- 33. Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69(2):381-387. [PubMed] [Google Scholar]
- 34. Eriksson P, Kallin B, van’t Hooft FM, Båvenholm P, Hamsten A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A. 1995;92(6):1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsantes AE, Nikolopoulos GK, Bagos PG, et al. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb Haemost. 2007;97(6):907–913. [PubMed] [Google Scholar]
- 36. Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ridker PM, Gadboury CL, Conlin PR, Seely EW, Williams GH, Vaughan DE. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation. 1993;87(6):1969–1973. [DOI] [PubMed] [Google Scholar]
- 38. Dilley A, Austin H, Hooper WC, et al. Relation of three genetic traits to venous thrombosis in an African-American population. Am J Epidemiol. 1998;147(1):30–35. [DOI] [PubMed] [Google Scholar]
- 39. González Ordóñez AJ, Fernández Carreira JM, Medina Rodríguez JM, et al. Risk of venous thromboembolism associated with the insertion/deletion polymorphism in the angiotensin-converting enzyme gene. Blood Coagul Fibrinolysis. 2000;11(5):485–490. [DOI] [PubMed] [Google Scholar]
- 40. Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8(1):1–21. [DOI] [PubMed] [Google Scholar]
- 41. Aguie GA, Rader DJ, Clavey V, et al. Lipoproteins containing apolipoprotein B isolated from patients with abetalipoproteinemia and homozygous hypobetalipoproteinemia: identification and characterization. Atherosclerosis. 1995;118(2):183–191. [DOI] [PubMed] [Google Scholar]
- 42. Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol. 2014;11(3):140–156. [DOI] [PubMed] [Google Scholar]