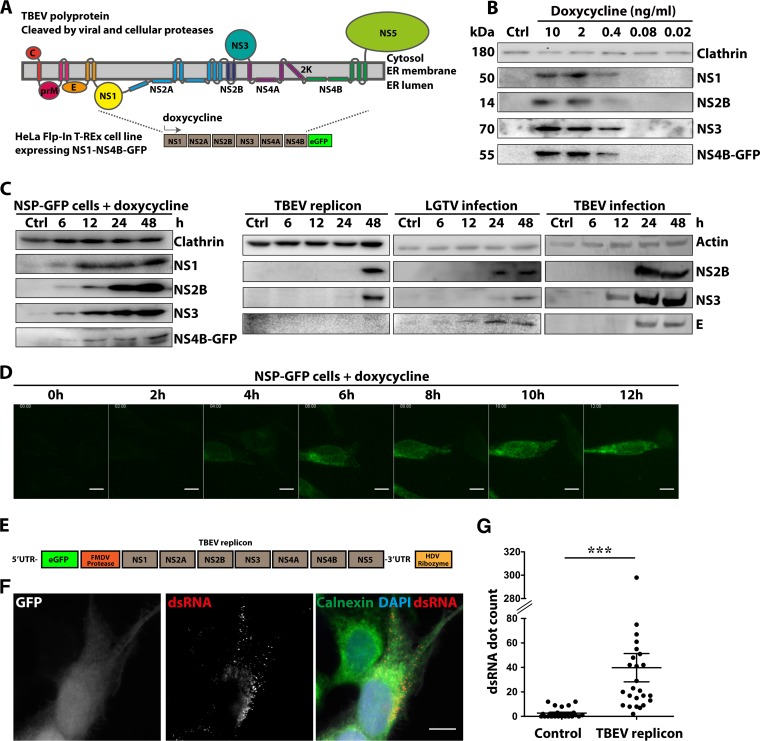
FIG 1.
Dose- and time-dependent expression of NS1-NS4B-GFP in Flp-In T-REx HeLa cells. (A) Schematic illustration of the proposed membrane topology of the TBEV polyprotein, with color-coded representation of the individual structural (C, prM, and E) and nonstructural (NS1 to NS5) proteins. The GFP-tagged polyprotein (NS1 to NS4B) expressed following dox addition in the constructed NSP-GFP-Flp-In cells is shown below. (B) Immunoblotting expression analysis of the individual NS proteins, as indicated, following induction of NSP-GFP cells with different concentrations (0 to 10 ng/ml) of dox. Clathrin served as a loading control. (C) Immunoblotting analysis of the time-dependent expression of NS proteins, as indicated, following induction with 2 ng/ml dox in NSP-GFP cells (left), transfection with the TBEV DNA replicon (1 μg DNA per 5 × 106 cells) (middle), or infection with LGTV (MOI of 1) or TBEV Torö strain (MOI of 1) (right). Actin and clathrin served as loading controls. (D) Representative fluorescence micrographs of the time-dependent expression of NS4B-GFP in NSP-GFP cells induced by 2 ng/ml dox, as recorded by live cell imaging. Scale bars = 10 μm. (E) Schematic illustration of the organization of the TBEV DNA replicon. (F) Representative images from immunofluorescence analysis of Flp-In HeLa cells transfected with the TBEV DNA replicon. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), and the ER marker calnexin was labeled with specific antibody, as indicated. dsRNA was labeled with the mouse anti-dsRNA monoclonal antibody J2. The GFP signal served as the reporter for replicon transfection. Scale bar = 10 μm. (G) Quantification of dsRNA in TBEV-replicon-transfected cells (n = 25) and untransfected cells (n = 25) from the immunofluorescence analysis in panel F. The dsRNA quantification was performed with Imaris v7.5 software (Bitplane). The statistical analysis was performed with GraphPad Prism software (GraphPad Software) (n = 25). ***, P ≤ 0.01 (t test).