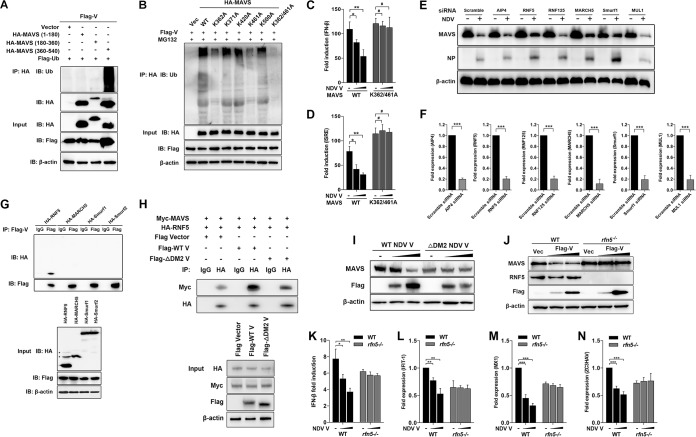
FIG 5.
Characterization of RNF5 as the E3 ubiquitin ligase for V-mediated MAVS degradation. (A) HEK-293T cells were cotransfected with Flag-V, Flag-ubiquitin, and either empty vector or truncated HA-MAVS aa 1 to 180, 180 to 360, and 360 to 540. At 24 hpt, cells were harvested, immunoprecipitated with anti-HA antibody, and further detected using immunoblot analysis with anti-ubiquitin or anti-HA antibody. Expression levels of the proteins were analyzed by immunoblot analysis of the lysates with anti-HA, anti-Flag, or anti-β-actin antibody. (B) HEK-293T cells were cotransfected with Flag-V, Flag-ubiquitin, and either empty vector, WT, or mutated HA-MAVS K362A, K371A, K420A, K461A, K500A, and K362/461A and maintained in the presence of the proteasome inhibitor MG132 (20 μM, 6 h prior to immunoprecipitation). At 24 hpt, cells were harvested, immunoprecipitated with anti-HA antibody, and further detected using immunoblot analysis with anti-ubiquitin antibody. Expression levels of the proteins were analyzed by immunoblot analysis of the lysates with anti-HA, anti-Flag, or anti-β-actin antibody. (C and D) HEK-293T cells were cotransfected with PRL-TK, HA-MAVS, or K362/461A MAVS and either empty vector (−) or Flag-V (1 or 2 μg/ml; wedge) as well as p-125Luc (C) or pISRE-luc (D). At 24 hpt, cells were harvested and assessed for luciferase activity. The results are presented as relative luciferase activity. (E) HeLa cells were transfected with either scrambled siRNA or specific siRNA targeting AIP4, RNF5, RNF125, MARCH5, Smurf1, or MUL1. At 48 h after transfection, cells were infected with NDV at an MOI of 1. Cells were harvested at 18 hpi and detected using immunoblot analysis with anti-MAVS, anti-NP, or anti-β-actin antibody. (F) The transfection experiments were performed as described for panel A. Cells were harvested and detected using qRT-PCR with primers for AIP4, RNF5, RNF125, MARCH5, Smurf1, or MUL1. (G) HEK-293T cells were cotransfected with Flag-V and HA-tagged RNF5, MARCH5, Smurf1, or Smurf2. At 24 hpt, cells were harvested, immunoprecipitated with anti-IgG or anti-Flag antibody, and further detected using immunoblot analysis with anti-HA or anti-Flag antibody. Expression levels of the proteins were analyzed by immunoblot analysis of the lysates with anti-HA, anti-Flag, or anti-β-actin antibody. (H) HEK-293T cells were cotransfected with Myc-MAVS, HA-RNF5, and either WT or ΔDM2 Flag-V vector. At 24 hpt, cells were harvested, immunoprecipitated with anti-IgG or anti-HA antibody, and further detected using immunoblot analysis with anti-Myc or anti-HA antibody. Expression levels of the proteins were analyzed by immunoblot analysis of the lysates with anti-HA, anti-Myc, anti-Flag, or anti-β-actin antibody. (I) HeLa cells were transfected with either WT or ΔDM2 Flag-V vector (1 or 2 μg/ml; wedge). At 12 hpt, cells were transfected with poly(I·C) (20 μg/ml). Cells were harvested at 12 hpt with poly(I·C) and detected using immunoblot analysis with anti-MAVS, anti-Flag, or anti-β-actin antibody. (J) WT and rnf5−/− HeLa cells were transfected with either vector or Flag-V (1 or 2 μg/ml; wedge). At 12 hpt, cells were transfected with poly(I·C) (20 μg/ml). Cells were harvested at 12 hpt with poly(I·C) and detected using immunoblot analysis with anti-MAVS, anti-RNF5, anti-Flag, or anti-β-actin antibody. (K) WT and rnf5−/− HeLa cells were cotransfected with p-125Luc, PRL-TK, and either vector or Flag-V (1 or 2 μg/ml; wedge). At 12 hpt, cells were transfected with poly(I·C) (20 μg/ml). Cells were harvested at 12 hpt with poly(I·C) and assessed for luciferase activity. The results are presented as relative luciferase activity. (L to N) The transfection experiments were performed as described for panel D. Cells were harvested at 12 hpt with poly(I·C) and detected using qRT-PCR with IFIT1 (L), MX1 (M), or ZC3HAV (N) primers.