Abstract
Complication of disseminated intravascular coagulation (DIC) is a determinant of the prognosis for patients with sepsis. The purpose of this study was to find DIC-related peptides in blood for prediction and early diagnosis of DIC in patients with sepsis. The participants were 20 patients with sepsis (age: 68.9 ± 11.4 years) and they were divided into 2 groups with (n = 8) and without (n = 12) a complication of DIC. Peptides in the serum of the patients were inclusively analyzed by a new method for peptidome analysis using a target plate, BLOTCHIP. By differential analysis of peptides in the blood from patients in the groups with and without DIC, we selected 13 mass spectrometry (MS) peaks as candidate marker peptides for prediction of DIC. By subsequent MS/MS structural analysis, 8 peptides were successfully identified as marker peptides for DIC in patients with sepsis. The peptides were fragments of serum amyloid A-2 protein, α2-HS-glycoprotein, fibrinogen α chain, fibrinogen β chain, serum albumin, collagen α1 (I) chain, collagen α1 (III) chain, and coagulation factor XIII A chain. In receiver–operating characteristic analysis for the relationships between the marker peptides and DIC, the area under the curve for each of these peptides was 0.594 to 0.760. We identified 8 blood marker peptides for prediction of DIC complication in patients with sepsis. Further studies by direct measurements of the serum peptide levels in larger numbers of patients with sepsis-induced DIC are needed to confirm the findings of this study.
Keywords: BLOTCHIP, disseminated intravascular coagulation, mass spectrometry, peptidome, sepsis
Background
It has been estimated that about 18 million patients suffer from severe sepsis each year worldwide,1 and the prognosis of patients is generally poor when they are complicated with circulatory failure: the mortality rate of such patients was reported to be 40% to 80% in the United States.2 Blood coagulation–fibrinolysis disorders such as disseminated intravascular coagulation (DIC), which frequently occurs in patients with sepsis, are involved in the pathogenesis of sepsis-induced circulatory failure3 and have a great effect on the prognosis of patients.4
Early goal-directed therapy is effective for patients with severe sepsis.5 Various markers related to coagulation–fibrinolysis disorders are used for the diagnosis of DIC. They include platelet count, fibrinogen concentration, prothrombin time (PT), antithrombin activity, and concentrations of thrombin–antithrombin complex, soluble fibrin, and prothrombin fragment 1+2.6 In addition, various kinds of cytokines including interleukin (IL)-1β, IL-6, IL-10, interferon-γ, vascular endothelial growth factor, tumor necrosis factor-α, and monocyte chemoattractant protein-1 have been shown to be elevated in patients with sepsis-associated DIC.7 Levels of plasminogen activator inhibitor-1 (PAI-1), IL-6, and IL-10 were higher, and protein C levels were lower in patients with sepsis having overt DIC than in those without overt DIC.8 These findings are consistent with the results of a study in which, using receiver–operating characteristic (ROC) analysis, protein C and PAI-1, as well as a complication with overt DIC, were shown to be significant prognostic factors for patients with sepsis.9 Moreover, protein C and presepsin have been shown to be the components of the optimal 2-marker panel for diagnosis of sepsis-induced DIC.10 However, there has been little information obtained by peptidome analysis on biomarkers that are useful for the prediction and early diagnosis of DIC in patients with sepsis.
The purpose of this study was therefore to find peptide markers in blood for prediction and early diagnosis of DIC complicated with sepsis. We performed a peptidome analysis using a new target plate, BLOTCHIP, that enables 1-step direct electric transfer of analytes from the 1-dimensional polyacrylamide gel electrophoresis (PAGE) gel to the target plate.11 In the peptidome analysis using BLOTCHIP, no pretreatment of blood samples is required, and thus peptides can be more efficiently detected, since removal of large amounts of blood proteins including albumin, which occurs prior to mass spectrometry (MS) in conventional peptidome analyses, is avoided in the method using BLOTCHIP. In the present study using this new method of peptidome analysis, we identified 8 peptides for prediction of DIC in patients with sepsis.
Patients and Methods
Patients and Blood Sample Collection
The participants were 20 patients (6 men and 14 women; mean age with its standard deviation: 68.9 ± 11.4 years) with sepsis defined as infection-induced systemic inflammatory response syndrome, for which diagnosis was performed using the criteria proposed by Bone et al.12 The causes of sepsis in the patients were pneumonia (n = 6, 3 patients with DIC and 3 patients without DIC), urinary tract infection (n = 2, 1 patient with DIC and 1 patient without DIC), meningitis (n = 1, without DIC), peritonitis (n = 2, 1 patient with DIC and 1 patient without DIC), cholangitis (n = 1, without DIC), ileus (n = 1, without DIC), lung injury (n = 1, with DIC), mediastinitis (n = 1, with DIC), cervical myelitis (n = 1, without DIC), necrotizing fasciitis (n = 2, without DIC), and mesenteric arterial occlusion (n = 1, with DIC). The cause of sepsis was unknown in 1 patient (without DIC). Blood samples were collected from the radial artery of each patient at the time of admission to the hospital (Department of Emergency, Disaster and Critical Care Medicine, Hyogo College of Medicine), and serum was separated and stored at −80°C until the time of peptidome analysis. Separated serum and plasma samples were also used for determination of inflammation and blood coagulation markers. Using EDTA-anticoagulated whole blood, blood cell counts were determined. The patients were divided into 2 groups with (n = 8) and without (n = 12) a complication of DIC during their clinical course after admission. We used the criteria by the International Society on Thrombosis and Haemostasis for diagnosis of DIC.13 The protocol of this study was approved by the Hyogo College of Medicine Ethics Committee (No. 1495 in 2015).
Peptidome Analysis
Serum peptidomic analysis was conducted using a newly established 1-step direct transfer technology, “BLOTCHIP-MS analysis”, as described elsewhere.11,14,15 Briefly, peptides and proteins in serum samples were separated using sodium dodecyl sulfate (SDS)-PAGE and then electroblotted onto BLOTCHIP (Protosera Inc, Amagasaki, Japan). Matrix-assisted laser desorption/ionization (MALDI) matrix, α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich Co, St. Louis, Missouri), was applied directly onto BLOTCHIP, and peptidome profiles were obtained in a linear mode of UltraFlexII TOF/TOF (Bruker Daltonics Inc, Billerica, Massachusetts). All sample measurements were repeated 4 times. Statistical analyses of MS spectral data for a comparison between the 2 patient groups (with and without DIC: DIC and non-DIC groups, respectively) were conducted. In this study, the peptides proposed as candidates of markers for DIC exist more or less in all of the patients, and the spectrum of each peptide was different among the patients in MS of the peptidome analysis and was compared between the DIC and non-DIC groups using a tool for spectrum data analysis, ClinProTools version 2.2 (Bruker Daltonics Inc).
Several sera containing high concentrations of target peptides were collected for peptide sequencing analyses. Peptides were extracted using Sep-Pak C18 solid-phase extraction cartridges (Waters Corporation, Milford, Massachusetts). The eluate was concentrated and applied to an ÄKTA purifier (GE Healthcare UK Ltd, Buckinghamshire, United Kingdom) that was equipped with a C18 silica-based column (COSMOSIL 5C18-AR-II; Nacalai Tesque, Inc, Kyoto, Japan). The fractionated eluate was analyzed using ultraflexII TOF/TOF 50 (Bruker Daltonics Inc) and a Thermo Scientific Q Exactive Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific Inc, Waltham, Massachusetts).
For peptide identification, MASCOT software version 2.1 was used for an “MS/MS ions search”. Parent peptide and MS/MS ion tolerance parameters were set at ±100 ppm and ±0.7 Da, respectively. The SwissProt sequence database (UniProtKB/Swiss-Prot), of which the taxonomy was limited to “humans”, was selected for the searches. The proteolytic enzyme parameter was set to “None”. “Oxidation”, “Deamidation,” and “Acetylation” were selected as variable modifications. The peptide identification criteria for this work were based on a probability-based MOWSE scoring algorithm, and the significant threshold was set to P < .05.
Determination of Blood Cell Counts and Inflammation and Blood Coagulation Marker Concentrations in Blood
Red blood cell, white blood cell, and platelet counts were measured by flow cytometry using Sysmex XN-1000 Automated Hematology Analyzer (Sysmex Corp, Kobe, Japan). C-reactive protein (CRP), fibrinogen, activated partial thromboplastin time (APTT), PT, and d-dimer were measured using the following commercial kits: CRP-Latex X2 (Denka Seiken Co, Ltd, Niigata, Japan), Thrombocheck Fib(L) (MBL Co, Ltd, Ina, Japan), Thrombocheck APTT-SLA (Sysmex Corp), Thromborel S (Sysmex Corp), and Hexamate D-dimer (MBL Co, Ltd), respectively.
Statistics
Statistical analyses of MS spectral data were conducted using ClinProTools version 2.2 (Bruker Daltonics). Other statistical analyses were conducted using a computer software program (SPSS version 16.0J for Windows, Chicago, Illinois). Some variables were compared between the DIC and non-DIC groups using unpaired Student t test or the Mann-Whitney U test. The relationship of the MS peak value of each peptide with DIC was investigated using ROC analysis. Spearman rank correlation coefficients of each peptide marker MS peak value with blood cell counts, inflammation markers, and blood coagulation markers were calculated. P values <.05 were defined as significant.
Results
Table 1 shows profiles of patient groups with and without a complication of DIC. The mean ages of the patients were not significantly different in the DIC and non-DIC groups. The median acute physiology and chronic health evaluation II (APACHE II) score was significantly higher in the DIC group than in the non-DIC group, while the median sequential organ failure assessment (SOFA ) score was not significantly different in the 2 groups. Red blood cell, white blood cell, and platelet counts were significantly lower in the DIC group than in the non-DIC group, and the level of d-dimer was significantly higher in the DIC group than in the non-DIC group. There were no significant differences in the levels of CRP, fibrinogen, and other blood coagulation markers including APTT, PT, and PT international normalized ratio (PT-INR).
Table 1.
Comparison of Clinical Data in the Patient Groups With and Without DIC.a
| Non-DIC, n = 12 | DIC, n = 8 | |
|---|---|---|
| Age | 67.7 ± 9.8 | 70.6 ± 13.9 |
| APACHE II score | 23.5 (15.5, 28.5) | 32.0 (25.0, 39.0)b |
| SOFA score | 4.50 (2.00, 5.75) | 5.00 (4.00, 13.25) |
| CRP, mg/dL | 15.2 (12.1, 25.9) | 26.6 (4.5, 36.7) |
| RBC, 104/μL | 393.0 (322.5, 419.0) | 296.0 (274.8, 350.3)c |
| WBC, 103/μL | 16.1 (11.9, 17.3) | 7.9 (3.5, 12.1)c |
| Platelets, 104/μL | 23.0 (16.9, 28.9) | 9.7 (5.8, 16.6)c |
| Fbg, mg/dL | 653.5 (484.0, 817.3) | 512.5 (366.3, 663.3) |
| APTT, seconds | 31.2 (27.9, 36.2) | 56.5 (26.6, 109.9) |
| PT, % | 63.8 (52.7, 73.7) | 58.0 (34.7, 76.1) |
| PT-INR | 1.27 (1.17, 1.40) | 1.35 (1.17, 1.84) |
| D-dimer, μg/mL | 2.08 (0.93, 4.12) | 5.15 (3.13, 9.05)b |
Abbreviations: APTT, activated partial thromboplastin time ; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; Fbg, fibrinogen; PT, prothrombin time; PT-INR, prothrombin time-international normalized ratio; RBC, red blood cell count; WBC, white blood cell count.
aMedians with the 25 and 75 percentile values (for the variables except for age) or means with standard deviations (for age) are shown. Significant differences from the non-DIC group are indicated as below.
b P < .05.
c P < .01.
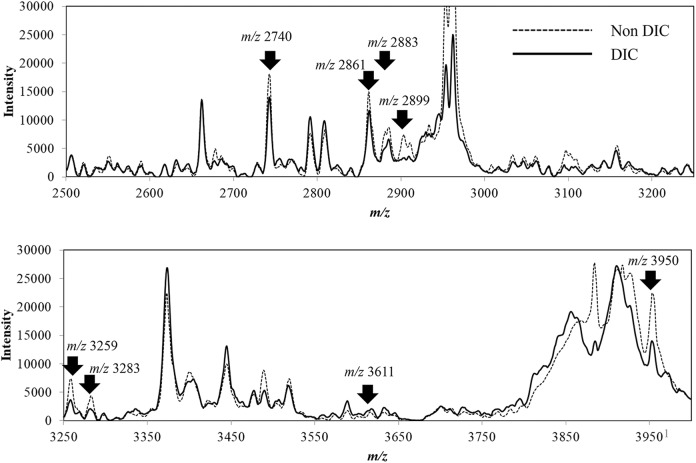
Figure 1 shows a superimposition of average MS spectra for each group obtained by matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) analysis of the serum peptides transferred to BLOTCHIP. By differential analysis using ClinProTools 2.2 software and BLOTMATE2.0 software, we found that levels of 13 peptides were statistically different in the 2 groups with and without DIC. Among the 13 peptides, as mentioned subsequently, we finally succeeded in identification of 8 DIC-related peptides (m/z 2740, 2861, 2883, 2899, 3259, 3283, 3611, and 3950 peptides), which are indicated by arrows in Figure 1.
Figure 1.
Differential profiling of serum sampled from patients with and without disseminated intravascular coagulation (DIC). Each integrated spectrum normalized with ClinPro Tools version 2.2 is the average of 8 samples (total of 32 integrations) from the DIC group and the average of 10 samples (total of 40 integrations) from the non-DIC group. The arrows indicate 8 spectrum peaks of the peptides that showed significant differences in the DIC and non-DIC groups and wereidentified by subsequent mass spectrometry (MS)/MS structural analysis.
To obtain more information about the peptides, we conducted MS/MS analysis of the serum peptides using Ultraflex II TOF/TOF mass spectrometer (Brucker Daltonics Inc) and quadrupole Orbitrap (Q Exactive) MS system (Thermo Fisher Scientific, Bremen, Germany). We tried to purify all of the 13 peptides, but only 8 were successfully purified by reverse-phase chromatography and harvested with a sufficient amount of each peptide for carrying out MALDI-TOF-MS/MS analysis. A MASCOT search revealed that m/z 2740, 2861, 2883, 2899, 3259, 3283, 3611, and 3950 peptides were fragments of α2-HS-glycoprotein, fibrinogen α chain, fibrinogen β chain, serum albumin, collagen α1 (III) chain, collagen α1 (I) chain, serum amyloid A-2 protein, and coagulation factor XIII A chain, respectively (Table 2).
Table 2.
Profiles of the Identified Peptide Fragments Associated With DIC in Sepsis Patients.
| Monoisotopic [M+H] | Origin of the Peptide | Amino Acid Number (N-/C-terminus) | Ions Score | Peptide Sequence (Modification) |
|---|---|---|---|---|
| 2739.5238 | α2-HS-glycoprotein | 341-367 | 51 | R.TVVQPSVGAAAGPVVPPCPGRIRHFKV.- |
| 2861.3373 | Fibrinogen α chain | 603-629 | 77 | K.MADEAGSEADHEGTHSTKRGHAKSRPV.R |
| 2882.5383 | Fibrinogen β chain | 45-71 | 45 | R.GHRPLDKKREEAPSLRPAPPPISGGGY.R |
| 2898.5625 | Serum albumin | 27-51 | 32 | A.HKSEVAHRFKDLGEENFKALVLIAF.A |
| 3259.4847 | Collagen α -1(III) chain | 652-687 | 97 | G.ENGKaPGEPGPaKGDAGAPaGAPaGGKGDAGAPaGERGPaPG.L |
| 3282.5622 | Collagen α -1(I) chain | 592-628 | 37 | E.PaGKAGERGVPaGPaPGAVGPAGKDGEAGAQbGPPaGPaAGPaA.G |
| 3610.7534 | Serum amyloid A-2 protein | 90-122 | 45 | R.GAEDSLADQAANKWGRSGRDPNHFRPAGLPEKY.- |
| 3949.9751 | Coagulation factor XIII A chain | 2-38 | 58 | M.SETSRTAFGGRRAVPPNNSNAAEDDLPTVELQGVVPR.G (acetyl (protein N-term)) |
a oxidated.
b deamidated.
The results of ROC analysis for the relationship of the MS peak of each peptide with occurrence of DIC are shown in Table 3. The area under the curve (AUC) in ROC analysis for the relationship of each peptide with DIC was 0.594 to 0.760 (P < .01). Among the peptides, the largest AUC was obtained by ROC analysis for the fragment of coagulation factor XIII A chain (m/z 3950) with sensitivity and specificity being 62.5% and 91.7%, respectively.
Table 3.
ROC Analysis for the Relationships Between Peptides and DIC in Patients With Sepsis.
| Monoisotopic [M+H] | AUC | Sensitivity (%) | Specificity (%) | Median Ratio (DIC/non-DIC)a | P Value |
|---|---|---|---|---|---|
| 2740 | 0.708 | 37.5 | 100 | 0.77 | <.01 |
| 2861 | 0.646 | 37.5 | 91.7 | 0.78 | <.01 |
| 2883 | 0.594 | 50.0 | 91.7 | 0.77 | <.01 |
| 2899 | 0.719 | 75.0 | 66.7 | 0.46 | <.01 |
| 3259 | 0.719 | 87.5 | 58.3 | 0.51 | <.01 |
| 3283 | 0.677 | 62.5 | 83.3 | 0.47 | <.01 |
| 3611 | 0.698 | 87.5 | 58.3 | 1.40 | <.01 |
| 3950 | 0.760 | 62.5 | 91.7 | 0.62 | <.01 |
Abbreviations: AUC, area under the curve; DIC, disseminated intravascular coagulation; ROC, receiver–operating characteristic.
a Median ratio of spectrum intensity in the DIC group to that in the non-DIC group.
Table 4 shows correlations of each peptide marker intensity obtained in MS analysis with blood cell counts, inflammation markers, and blood coagulation markers. Neither CRP nor fibrinogen showed a significant correlation with any of the peptide markers. m/z 2883 was significantly correlated with APTT, PT, PT-INR, and red blood cell count, while there was no correlation between m/z 2883 and fibrinogen. m/z 2899, m/z 3259, m/z 3283, and m/z 3950 were significantly correlated with red blood cell and platelet counts. There was also a significant correlation between m/z 2899 and APTT. On the other hand, m/z 2740, m/z 2861, and m/z 3611 were not significantly correlated with any of the abovementioned inflammation- and coagulation-related variables and blood cell counts measured in this study.
Table 4.
Correlations Between Each Peptide Spectrum Intensity and Levels of Blood Cell Count and Variables Related to Inflammation and Blood Coagulation.a
| m/z | CRP | RBC | WBC | Platelets | Fbg | APTT | PT | PT-INR | d-dimer |
|---|---|---|---|---|---|---|---|---|---|
| 2740 | −0.257 | 0.383 | 0.042 | 0.054 | −0.156 | −0.266 | 0.370 | −0.370 | −0.081 |
| 2861 | −0.208 | 0.341 | −0.096 | −0.060 | −0.247 | −0.254 | 0.253 | −0.234 | −0.009 |
| 2883 | −0.200 | 0.505b | −0.003 | 0.412 | −0.013 | −0.558b | 0.566c | −0.541b | 0.185 |
| 2899 | −0.262 | 0.618c | 0.317 | 0.499b | 0.289 | −0.597c | 0.317 | −0.314 | −0.056 |
| 3259 | −0.307 | 0.600c | 0.047 | 0.480b | −0.017 | −0.432 | 0.368 | −0.354 | 0.215 |
| 3283 | −0.041 | 0.463b | 0.153 | 0.450b | 0.181 | −0.307 | 0.393 | −0.387 | −0.045 |
| 3611 | 0.305 | −0.310 | −0.353 | −0.250 | −0.209 | 0.405 | −0.320 | 0.352 | 0.308 |
| 3950 | −0.096 | 0.576c | 0.038 | 0.532b | 0.006 | −0.212 | 0.354 | −0.355 | −0.125 |
Abbreviations: APTT, activated partial thromboplastin time; CRP, C-reactive protein; Fbg, fibrinogen; PT, prothrombin time; PT-INR, prothrombin time-international normalized ratio; RBC, red blood cell count; WBC, white blood cell count.
a Spearman rank correlation coefficients between each pair of the variables are shown.
b P < .05, significant coefficient.
c P < .01, significant coefficient.
Discussion
We identified 8 peptides that were significantly changed in relation to DIC in the blood of patients with sepsis. This is, to the best of our knowledge, the first study in which inclusive peptidome analysis was performed with the aim of finding peptide markers for sepsis-induced DIC. Among the 8 peptides, 3 peptides are fragments of coagulation-related peptides, fibrinogen α chain, fibrinogen β chain, and coagulation factor XIII A chain. Thus, it is plausible that these peptides are associated with the occurrence of DIC, a severe coagulation disorder, in patients with sepsis. In fact, various coagulation-related markers as well as inflammatory mediators have been used and proposed for diagnosis of DIC and prediction of its prognosis as mentioned earlier.6-10 Another peptide (m/z 3611 peptide) that was changed in the serum of patients with DIC is a fragment of serum amyloid A-2 protein, which is known as an acute-phase protein.16 Among the DIC-related peptides identified in the present study, m/z/ 3611 peptide was the only peptide that was positively associated with occurrence of DIC (see ratios of DIC/non-DIC in Table 3). Thus, an increase in the level of this peptide might reflect the relation of acute-phase reaction with DIC in patients with sepsis, since the degree of acute-phase reaction is speculated to be stronger in patients with sepsis complicated with DIC than in patients having sepsis without DIC.
We also detected a fragment of α2 HS glycoprotein (m/z 2740) that was decreased in the serum of patients with DIC. α2 HS glycoprotein, called fetuin-A, has been shown to be involved in glucose metabolism, bone metabolism, and immune regulation.17 A change in the blood level of fetuin A has recently been reported to be a predictor for prognosis of patients with sepsis.18 Moreover, administration of fetuin-A to mice with sepsis has been shown to result in improvement of prognosis with a decrease in high mobility group box 1 protein (HMGB1),19 which is a nuclear mediator of inflammation, and has been reported to be increased in sepsis-induced DIC.20 Since complication with DIC is a determinant of the prognosis in patients with sepsis, the finding in the abovementioned study using an animal model of sepsis agrees with the inverse association between the fragment peptide of α2HS glycoprotein and DIC found in the present study. The other 3 peptides identified in this study were fragments of albumin and collagen α (I) and (III) chains, which might be related to malnutrition and tissue damage, respectively, in patients with sepsis-induced DIC.
Neither CRP nor fibrinogen showed a significant correlation with any of the peptide markers, suggesting that the associations of the peptide markers with DIC are independent of the degree of inflammation in patients with sepsis. The associations of m/z 2883 with blood coagulation markers seem reasonable, since m/z 2883 is a fragment of fibrinogen β chain. A common mechanism, proteolysis, for generation of m/z 2883 and activation of blood coagulation may explain the associations between m/z 2883 and the abovementioned blood coagulation markers. m/z 2899, m/z 3259, m/z 3283, and m/z 3950 were associated with blood counts of red blood cells and platelets. m/z 2899 is a fragment of albumin, and both m/z 3259 and m/z 3283 were fragments of collagen. Thus, the associations between these peptides and blood counts of red blood cells and platelets might be mediated via the nutritional status and tissue damage of patients. On the other hand, m/z 2740 (a fragment of α2-HS-glycoprotein) and m/z 3611 (a fragment of serum amyloid A-2 protein) were not significantly correlated with any of the abovementioned variables measured in this study and were therefore thought to be inflammation- and hemostasis-independent markers for prediction of DIC in patients with sepsis.
There are some limitations in this study. The number of patients was small, and there were various causes of sepsis in the patients. Thus, this study is a pilot study for finding candidate peptides for early prediction of DIC in patients with sepsis. Further studies using a larger number of patients and adjusting backgrounds of the patients are needed to confirm the findings of this study and determine the significance of each peptide. Serum samples for peptidome analysis were collected from the patients only at the time of admission to the hospital, and follow-up peptidome analysis using later serum samples was not performed. It would be interesting to investigate the time courses of serum peptide levels and determine the time-dependent relationships between peptide levels and blood markers of coagulation disorders in patients with sepsis-induced DIC in future studies. In the present study, we were able to identify 8 peptides among 13 peptides for which the serum levels were found to be significantly different in the DIC and non-DIC groups, and further studies are needed to identify the other 5 peptides.
Conclusion
Eight peptides, fragments of serum amyloid A-2 protein, α2-HS-glycoprotein, fibrinogen α chain, fibrinogen β chain, serum albumin, collagen α1 (I) chain, collagen α1 (III) chain, and coagulation factor XIII A chain were identified as candidates markers for prediction of DIC complication in patients with sepsis. Early diagnosis and intervention for sepsis-induced DIC are important for improving the prognosis of patients with sepsis. Further studies using a larger number of patients and adjusting their backgrounds are needed to confirm the findings of this study and elucidate their clinical significance.
Footnotes
Authors’ Note: IW organized the study and contributed to study design and manuscript preparation. NM and TU contributed to sample collection. DN and LL contributed to peptidome analysis, and KT contributed to peptidome analysis and manuscript preparation. JK contributed to study design and sample collection.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in-Aid for Scientific Research (No. 26670794) from the Japan Society for the Promotion of Science.
References
- 1. Slade E, Tamber PS, Vincent JL. The surviving sepsis campaign: raising awareness to reduce mortality. Crit Care. 2003;7(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartemink KJ, Hack CE, Groeneveld AB. Relation between coagulation/fibrinolysis and lactate in the course of human septic shock. J Clin Pathol. 2010;63(11):1021–1026. [DOI] [PubMed] [Google Scholar]
- 4. Dahmash NS, Chowdhury NH, Fayed DF. Septic shock in critically ill patients: aetiology, management and outcome. J Infect. 1993;26(2):159–170. [DOI] [PubMed] [Google Scholar]
- 5. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 6. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11(4):761–767. [Google Scholar]
- 7. Walborn A, Hoppensteadt D, Syed D, Mosier M, Fareed J. Biomarker profile of sepsis-associated coagulopathy using biochip assay for inflammatory cytokines. Clin Appl Thromb Hemost. 2018;24(4):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoppensteadt D, Tsuruta K, Hirman J, Kaul I, Osawa Y, Fareed J. Dysregulation of inflammatory and hemostatic markers in sepsis and suspected disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2015;21(2):120–127. [DOI] [PubMed] [Google Scholar]
- 9. Umemura Y, Yamakawa K, Kiguchi T, et al. Design and evaluation of new unified criteria for disseminated intravascular coagulation based on the Japanese Association for Acute Medicine criteria. Clin Appl Thromb Hemost. 2016;22(2):153–160. [DOI] [PubMed] [Google Scholar]
- 10. Ishikura H, Nishida T, Murai A, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care. 2014;18(1):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka K, Tsugawa N, Kim YO, Sanuki N, Takeda U, Lee LJ. A new rapid and comprehensive peptidome analysis by one-step direct transfer technology for 1-D electrophoresis/MALDI mass spectrometry. Biochem Biophys Res Commun. 2009;379(1):110–114. [DOI] [PubMed] [Google Scholar]
- 12. Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Methylprednisolone severe sepsis study group. Crit Care Med. 1989;17(5):389–393. [PubMed] [Google Scholar]
- 13. Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 14. Araki Y, Nonaka D, Tajima A, et al. Quantitative peptidomic analysis by a newly developed one-step direct transfer technology without depletion of major blood proteins: its potential utility for monitoring of pathophysiological status in pregnancy-induced hypertension. Proteomics. 2011;11(13):2727–2737. [DOI] [PubMed] [Google Scholar]
- 15. Uchiyama K, Naito Y, Yagi N, et al. Peptidomic analysis via one-step direct transfer technology for colorectal cancer biomarker discovery. J Proteomics Bioinform. 2015. doi: 10.4172/jpb.S5-005. [Google Scholar]
- 16. Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–523. [DOI] [PubMed] [Google Scholar]
- 17. Mori K, Emoto M, Inaba M. Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(2):124–146. [DOI] [PubMed] [Google Scholar]
- 18. Karampela I, Kandri E, Antonakos G, et al. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: a prospective study. J Crit Care. 2017;41:78–85. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE. 2011;6(2):e16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatada T, Wada H, Nobori T, et al. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94(5):975–979. [DOI] [PubMed] [Google Scholar]