Abstract
Venous thromboembolism (VTE) is a highly morbid condition with several available oral anticoagulant treatment options. Numerous studies have been published comparing warfarin to direct oral anticoagulants; however, several populations remain underrepresented in these reports. We surveyed members of The Venous ThromboEmbolism Network U.S. working group regarding their oral anticoagulant preferences for the treatment of VTE in different and challenging populations. In individuals with VTE and no other medical comorbidities, respondents preferred either rivaroxaban (48.7%) or apixaban (48.7%). Apixaban (53.3%) was preferred in elderly individuals with an increased risk of bleeding. Warfarin was preferred in individuals with liver or kidney dysfunction (42% and 47%), altered metabolism (>55%), and antiphospholipid antibody syndrome (84.2%). Low-molecular-weight heparin was preferred in individuals with malignancy (56.6%), followed by edoxaban (23.7%). These findings may help guide clinicians when choosing an anticoagulant in these challenging situations and demonstrate the urgent need for additional study in these groups.
Keywords: anticoagulants, deep vein thrombosis, factor Xa inhibitors, low-molecular-weight heparins, lupus inhibitor, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a major cause of morbidity and mortality across all populations.1 Until recently, warfarin was the only oral anticoagulant available for the treatment and secondary prevention of VTE. Over the past decade, direct oral anticoagulants (DOACs) including apixaban,2 rivaroxaban,3,4 dabigatran,5,6 and edoxaban,7 have emerged as alternatives to warfarin for the treatment of VTE which do not require routine monitoring or frequent dose adjustments. When compared to warfarin, DOACs have been found to be equally efficacious and are generally associated with less bleeding.2–7 Presently, there are no published head-to-head trials comparing one DOAC to another.
There are several populations in which the effectiveness of DOACs is uncertain due to their underrepresentation in clinical trials. Only recently have prospective studies become available to support the use of edoxaban8 and possibly rivaroxaban9 in patients with cancer-associated thrombosis (CAT). Several ongoing clinical trials are assessing use of DOACs among patients with CAT, antiphospholipid syndrome (APS), obesity, and in children. Uncertainty exists regarding the use of DOACs in patients with renal and/or liver insufficiency or who are taking other interacting medications. Despite the recent shift in oral anticoagulant preference toward DOACs, circumstances remain where providers may continue to prescribe warfarin or low-molecular-weight heparin (LMWH).10,11
With the large amount of data being published on DOAC use, selecting the most appropriate anticoagulant for individual patients is increasingly challenging. Even when new evidence is identified as practice changing, it can take time for data to translate into changes in clinical practice.
The Venous ThromboEmbolism Network U.S. (VENUS) is a collaborative working group administered by Hemostasis and Thrombosis Research Society and sponsored by the Anticoagulation Forum, National Blood Clot Alliance, and American Society of Hematology (ASH) Foundation. The Venous ThromboEmbolism Network U.S. membership includes US pediatric and adult thrombosis specialists collaborating to design and conduct investigator-initiated studies to inform important questions regarding the prevention and management of thrombosis. This survey-based study among VENUS members reports the oral anticoagulant preferences of thrombosis specialists across a variety of clinical scenarios with the goal of delivering guidance to practicing clinicians.
Methods
Physician members of the VENUS network were queried using an online survey tool designed to assess opinions regarding the preferred oral anticoagulant to treat VTE in various patient scenarios. The 17-question survey included 11 patient scenarios addressing potential factors that would influence treatment choice including obesity (weight >125 kg), history of bleeding, altered drug absorption/drug interactions, renal or hepatic disease, APS, and metastatic cancer. Respondents were also asked to rank 9 provided considerations in the order in which they influence oral anticoagulant selection. Additional questions that addressed the demographics of respondents included clinical practice patient population (adult, pediatric, or both), practice location, and years of experience.
This study was reviewed and approved by the Institutional Review Board of the Medical College of Wisconsin. The survey was discussed at VENUS in-person meetings and administered electronically via a web-based survey interface (SurveyMonkey). The survey was sent by e-mail to the entire membership (127 physicians). Statistical analysis was descriptive, and Fisher exact tests were used to evaluate associations between responses to specific clinical scenarios and reported influencing and demographics factors. Bonferroni correction was used to adjust for multiple comparisons. With a starting α of .05 and considering 5 potentially significant tests, we adjusted the P value required for statistical significance to .01. Factors influencing oral anticoagulant recommendations were ranked based on the likelihood of respondents identifying them as having greater influence on their anticoagulant selection.
Results
Among 127 members surveyed, 80 (63%) responded. Fifty (62%) respondents reported treating exclusively adult patients, 23 (29%) reported treating exclusively pediatric patients, and the 7 (8%) remaining respondents reported treating both. Inclusion of the pediatric providers’ responses in the analysis did not significantly change the pattern of responses for the whole group. The experience level of the respondents was evenly distributed with 12 (21.5%) practicing for <5 years, 17 (30%) practicing for 5 to 10 years, 10 (17.5%) practicing for 10 to 15 years, and 17 (30%) practicing for more than 15 years. Respondents represented several regions of the United States with 17 (30%) practicing in the Northeast, 17 (30%) practicing in the Midwest, 12 (21%) practicing in the South, 2 (3.5%) practicing in the Northwest, and 9 (15.5%) practicing in the Southwest or California. Neither years of experience nor geographical location of practice influenced oral anticoagulant selection to a significant degree.
Individuals with VTE Without Other Medical Comorbidities
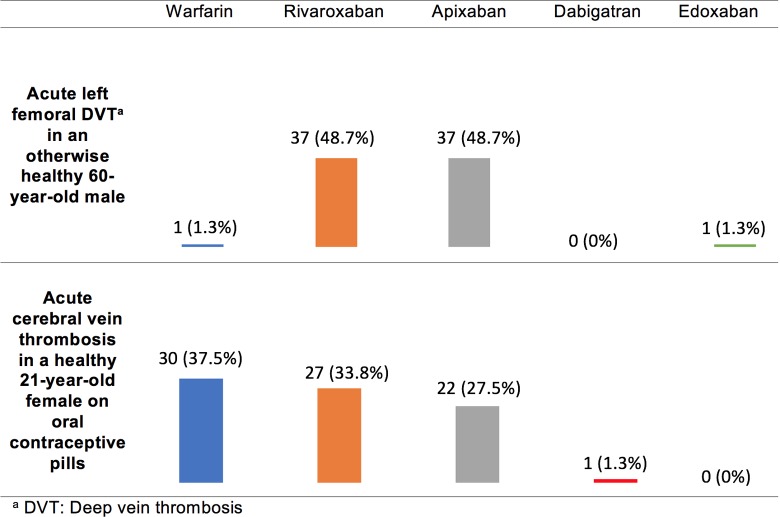
The responses to clinical vignettes regarding VTE treatment in individuals without other medical comorbidities are provided in Figure 1. Most (97%) respondents recommend a DOAC for the treatment of patients with VTE with acute lower extremity deep vein thrombosis (DVT). Rivaroxaban and apixaban were the preferred DOACs and were recommended in an identical proportion (48.7%). There was a trend toward preference of rivaroxaban over apixaban among respondents who selected insurance coverage as the most important consideration influencing decision-making, but this was not significant after Bonferroni correction (P = .021). Respondents were more likely to select warfarin than a DOAC for the treatment of females with cerebral vein thrombosis taking oral contraceptive pills (OCPs). Of respondents who recommend a DOAC, the selection of rivaroxaban and apixaban was comparable (33.8% versus 27.5%). There were no notable variables that predicted selection of warfarin versus a DOAC in this vignette.
Figure 1.
Survey responses for individuals with venous thromboembolism (VTE) and no other medical comorbidities.
Age/Organ Dysfunction
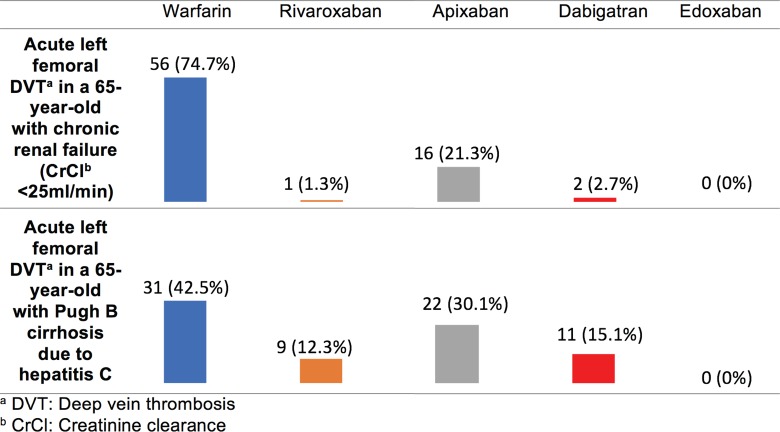
Figure 2 demonstrates that among individuals with renal insufficiency (CrCl <25 mL/min) or hepatic insufficiency (Pugh B cirrhosis), respondents were most likely to select warfarin as their preferred anticoagulant. Respondents who did select a DOAC were most likely to choose apixaban. There were no notable variables that predicted selection of warfarin versus a DOAC in these vignettes.
Figure 2.
Survey responses for individuals with organ dysfunction and venous thromboembolism (VTE) .
Increased Bleeding Risk
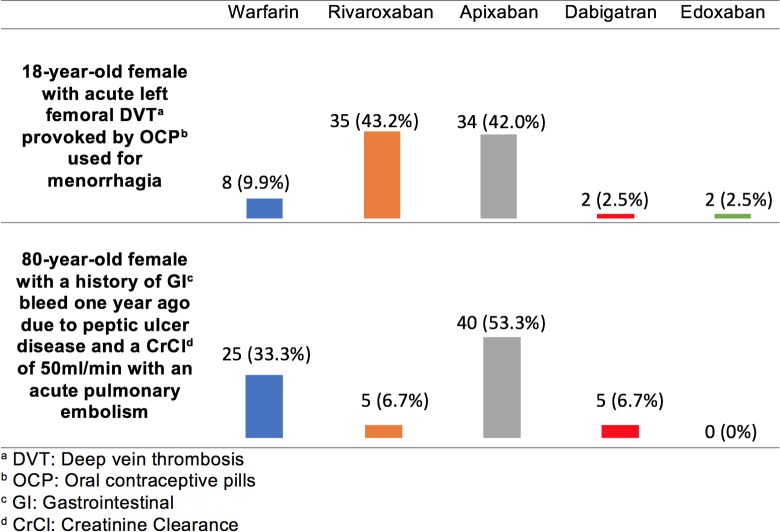
In the scenario of an 18-year-old female developing an acute DVT while taking combined OCPs for menorrhagia, the treatment selection was similar for patients without other medical comorbidities. Most respondents chose either apixaban (42.0%) or rivaroxaban (43.2%; Figure 3). In the treatment of acute pulmonary embolism in an 80-year-old with a CrCl of 50 mL/min and a history of GI bleed, respondents favored apixaban (53.5%) followed by warfarin (33.3%; Figure 3). In both scenarios, no variables significantly predicted respondents’ selections, although there was a trend toward adult practitioners being more likely to select apixaban when compared to pediatric providers who were more likely to select warfarin for an elderly patient with a history of GI bleeding (P = .011).
Figure 3.
Survey responses for individuals with perceived increased bleeding risk and venous thromboembolism (VTE) .
Altered Metabolism
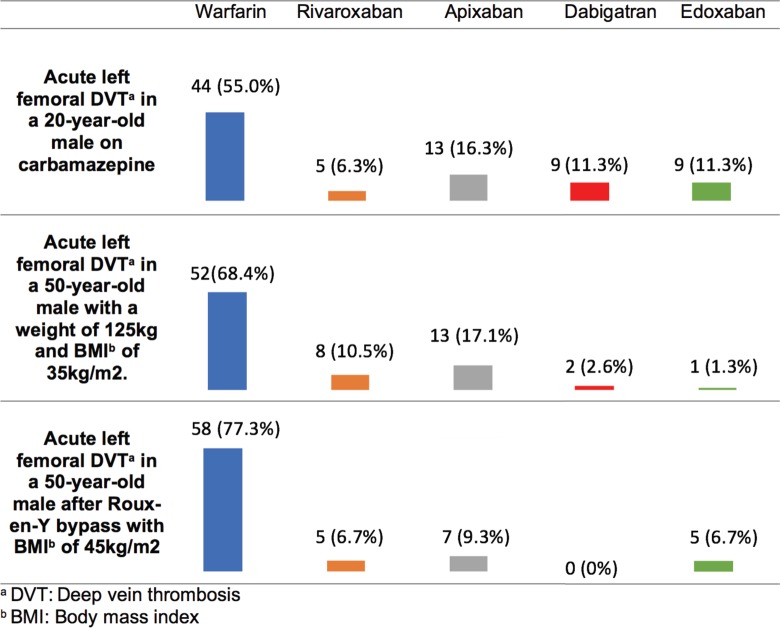
Among individuals with altered drug metabolism related to concomitant medications, obesity, or gastrointestinal surgery, respondents preferred warfarin over DOACs (Figure 4). Of those who selected DOACs, there was no clear preference for a given DOAC. Adult providers were more likely to select warfarin for an 18-year-old treated with carbamazepine compared to pediatric providers who were more likely to select a DOAC (P = .011). There were no notable predictor variables for the other 2 vignettes in this group.
Figure 4.
Survey responses for individuals with altered metabolism and venous thromboembolism (VTE) .
Other Populations
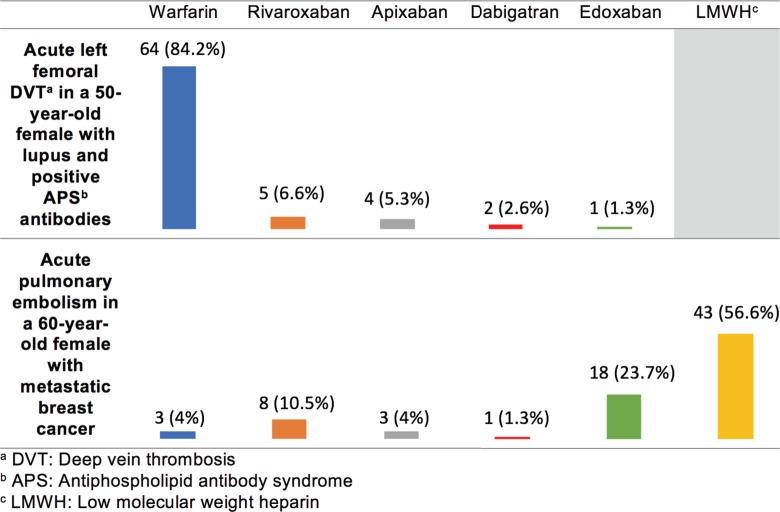
For the treatment of thrombosis among patients with APS, most respondents selected warfarin (84.2%) over a DOAC (15.8%; Figure 5). In the treatment of CAT, most respondents selected subcutaneous LMWH (56.6%) followed by edoxaban (23.7%). Respondents who selected edoxaban for the treatment of CAT were more likely to have read the abstracts of the Select-D and/or Hokusai VTE Cancer trials8,9 published just before the release of this survey and felt the results were practice changing (P < .001).
Figure 5.
Survey responses for individuals with antiphospholipid syndrome (APS) or active malignancy and venous thromboembolism (VTE).
Factors Influencing Oral Anticoagulant Recommendation
When asked to rank factors that most influence oral anticoagulant selection, most respondents felt that a patient’s insurance coverage, liver and kidney function, and bleeding risk were most influential with 27.6%, 24.7%, and 21.8% of respondents, respectively, choosing these factors as their top priority. Respondents identified P-gp or CYP3A inhibitor drug use, the need for LMWH, and the dose frequency (once versus twice per day) as being of intermediate importance in influencing oral anticoagulant recommendations. Lowest priority was given to pregnancy risk, availability of a reversal agent, or approval of prophylactic dosing after 6 months. The ranking of these factors by respondents did not significantly predict oral anticoagulant preferences among the vignettes.
Discussion
Between 2010 and 2015, four DOACs were approved by the US Food and Drug Administration (FDA) for the treatment of VTE, resulting in increased choice and complexity of VTE therapy. Major guidelines have expanded to include DOACs and, in certain cases, have recommended them over warfarin,12 but uncertainty and variation in the choice and use of these drugs persist. In the absence of clinical trials with head-to-head comparison among DOACs, choices between agents are based on patient preferences, financial considerations, and an understanding of the varying characteristics of each drug. This study aimed to better define the pattern of physician practices and the factors influencing them.
Rivaroxaban and apixaban were preferred for patients with DVT and no other medical comorbidities. Interestingly, only a single provider reported a preference for dabigatran or edoxaban. Possible reasons for this include the need for LMWH bridging and the relative lack of experience with edoxaban. The infrequent selection of dabigatran may seem somewhat surprising, as it is the only DOAC with an available reversal agent13; however, low priority was given to “availability of a reversal agent” as a consideration in respondent decision-making. We hypothesize that thrombosis providers understand the published evidence suggesting equal or improved outcomes in patients who have bled with DOAC therapy compared to warfarin,14 thus putting less of an emphasis on ability to reverse the anticoagulant effect of the medication. Preference for warfarin in patients with cerebral vein thrombosis is speculated to be at least partially associated with the lack of dedicated studies in using DOACs in patients with rare thromboses, including cerebral vein thrombosis and/or splanchnic vein thrombosis.
A preference for warfarin was evident in scenarios with abnormal drug clearance. Among DOACs, the preference for apixaban in patients with CrCl <25 mL/min may reflect FDA labeling that permits use in patients on dialysis.15 In addition, post hoc analyses of patients in the ARISTOTLE trial16 with impaired renal function (CrCl <50 mL/min) who were administered apixaban demonstrated equivalent or superior safety and efficacy, when compared to patients with normal renal function. Meta-analyses of DOACs versus vitamin K antagonists in patients with CrCl of 30 to 50 mL/min17 also suggest equivalence, but this level of renal function was not addressed in our survey. Following warfarin, apixaban was also the second most popular choice for patients with cirrhosis. This scenario was associated with the highest rate of dabigatran use, potentially due to the availability of a reversal agent, the fact that dabigatran is the least reliant on liver metabolism, or a preference for direct thrombin inhibition in patients with decreased clotting factor synthesis.
Warfarin was a common choice in situations involving altered drug metabolism, absorption, or distribution. While warfarin, apixaban, and rivaroxaban are all metabolized by the enzyme CYP3A4, the ability to easily measure the level of anticoagulation with warfarin likely lead to the preference of warfarin in these patients. Despite a meta-analysis of phase III trials suggesting that weight does not impact the efficacy of DOACs,18 warfarin remained the agent of choice among respondents in scenarios of patients with morbid obesity and Roux-en-y bypass. This decision may reflect International Society of Thrombosis and Haemostasis (ISTH) guidelines that recommend avoidance of DOACs in patients >120 kg due to the lack of high-quality evidence, the limited number of patients at extremes of weight in phase III trials, and practical experience in this patient population.19 Only a few patients at extremes of weight were included in registry trials or pharmacokinetic/pharmacodynamic studies.19 Assays to measure drug levels of the DOACs are not widely available, and therapeutic target levels are not well established, leaving uncertainty regarding what if any role DOAC monitoring or dose adjustment may play among patients with extremes of body weight or among those with organ dysfunction.
In a young female with menorrhagia requiring anticoagulation for VTE, respondents equally favored the DOACs rivaroxaban and apixaban over other options. This result is in spite of recent evidence demonstrating a predisposition to heavy menstrual bleeding (HMB) in patients taking rivaroxaban when compared to warfarin and possibly also apixaban; however, such studies have included small number of participants.20,21 This is an important area for further investigation, as patients who experience HMB on therapeutic anticoagulation may also be at higher risk for recurrent VTE due to intermittent drug interruptions.20
In patients with APS, warfarin is clearly favored by majority of respondents. This likely reflects a paucity of evidence and increased caution around use of DOACs in patients perceived to be highly hypercoagulable.22,23 A number of ongoing trials seek to study the efficacy of DOACs such as rivaroxaban and apixaban in patients with APS. Responses to the metastatic cancer patient scenario were influenced by the new data from the Select-D9 and the Hokusai VTE Cancer studies8 comparing dalteparin with rivaroxaban and edoxaban, respectively. These data were presented at the 2017 ASH Annual Meeting. Previously, LMWH was the agent of choice for CAT12 due to its established superiority over warfarin.10,11 Respondents who favored edoxaban over LMWH had not only read the abstracts but also felt that the results of these studies were practice changing. The rationale for preference of edoxaban over rivaroxaban is less clear but may reflect the comparative size of the Hokusai trial (1050 patients) versus Select-D (406 patients) and the lack of power to detect statistical significance in the Select-D trial.
Our study has several limitations and may not reflect practice among all providers. Only thrombosis providers were included in this study, but anticoagulation is frequently prescribed and managed in the primary care settings and by other subspecialists, such as cardiologists or pulmonologists, who were not included in our survey. Pediatric providers were included in this analysis, despite most questions being directed toward an adult population. While there was no significant difference between adult and pediatric provider responses, the inclusion of pediatric provider responses led to more heterogeneity of oral anticoagulant preferences. Pediatric provider responses may have been biased by the lack of an option to select LWMH in most vignettes and lack of FDA approval for use of DOACs in patients under 18 years of age. No scenarios addressing the use of DOACs in atrial fibrillation were included, which limits the applicability outside of VTE. Specific reasoning behind each choice was not solicited; therefore, the rationale for each choice could only inferred.
Our study has a number of strengths. We had a relatively high response rate of 63%. We included providers with not only a wide range of experience but also from various geographic locations, thereby reflecting a diversity of practice. Our study confirms that physicians specializing in VTE, while comfortable using DOACs in an otherwise uncomplicated scenario, more frequently select warfarin in populations with organ dysfunction and in cases with impaired drug metabolism or absorption. These observations provide direction for future clinical trials in key populations for whom the risk–benefit profile of DOACs remains uncertain.
In summary, while these scenarios lack provider preference homogeneity or evidence-based answers, preferences for a single agent were seen in most of the questions. Rivaroxaban and apixaban were selected equally in patients with VTE without other comorbidities. Warfarin was preferred in patients with renal or hepatic dysfunction, morbid obesity, and APS as well as in situations with potentially altered absorption or drug–drug interactions. By demonstrating heterogeneity and preference for warfarin in many common scenarios, this study highlights the need for further exploration and larger, randomized controlled studies of DOAC use in special populations including the morbidly obese, those with renal and liver dysfunction, those on interacting medications, and those with rare thrombosis or APS. It further highlights the need for multicenter studies coordinated by groups such as VENUS to successfully accrue a sufficient number of special population patients to address important questions. Treatment of VTE has changed with the availability of DOACs, and our data suggest that uncertainty remains in many scenarios. Practice will likely continue to evolve as new evidence emerges and providers should remain mindful as we gain experience with these agents in both adults and children.
Footnotes
Authors’ Note: Ethical approval to report this case series was obtained from the Institutional Review Board of the Medical College of Wisconsin (Approval Number: PRO00031090). Informed consent was not obtained because no patient information was published herein. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by, HRSA, HHS, or the US Government.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Woller served as a panelist for the American College of Chest Physicians (ACCP) Antithrombotic Guideline, 10th Edition. Tzu-Fei Wang, MD, is a site investigator for a phase 3 trial of rivaroxaban in children sponsored by Bayer. Rachel Rosovsky, MD MPH has been involved clinical trial research for both Bristol-Myers Squibb and Janssen pharmaceuticals. She also serves on the advisory board for Portola and is a consultant for BMS.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Scott Woller, MD, received investigator-initiated grant funding from Bristol-Myers-Squibb. Statistical support for this study was provided by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number 2H30MC24049, Mountain States Hemophilia Network.
ORCID iD: Genevieve Claire Moyer  http://orcid.org/0000-0003-1647-2515
http://orcid.org/0000-0003-1647-2515
Scott Woller  http://orcid.org/0000-0002-2522-2705
http://orcid.org/0000-0002-2522-2705
References
- 1. Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112(2):255–263. [DOI] [PubMed] [Google Scholar]
- 2. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. [DOI] [PubMed] [Google Scholar]
- 3. Bauersachs R, Berkowitz SD, Brenner B, et al. ; Einstein Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. [DOI] [PubMed] [Google Scholar]
- 4. Buller HR, Prins MH, Lensin AW, et al. ; Einstein-Pe Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. [DOI] [PubMed] [Google Scholar]
- 5. Schulman S, Kearon C, Kakkar AK, et al. ; Re-cover study group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. [DOI] [PubMed] [Google Scholar]
- 6. Schulman S, Kakkar AK, Goldhaber SZ, et al. ; Re-Cover II Trial Investigators. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. [DOI] [PubMed] [Google Scholar]
- 7. Buller HR, Decousus H, Grosso MA, et al. ; Hokusai VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. [DOI] [PubMed] [Google Scholar]
- 8. Raskob GE, Van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2017;378(7):615–624. [DOI] [PubMed] [Google Scholar]
- 9. Young A, Marshall A, Thirwall J, et al. Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the select-D pilot trial. Blood. 2017;130(Suppl 1):625. [DOI] [PubMed] [Google Scholar]
- 10. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 11. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs Warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. [DOI] [PubMed] [Google Scholar]
- 12. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 13. Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–520. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, Schulman D, Dowlatshahi A., et al. Direct oral anticoagulant- or warfarin-related major bleeding: characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest. 2017;152(1):81–91. [DOI] [PubMed] [Google Scholar]
- 15. Eliquis (apixaban) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. Princeton NJUaPINY, New York 10017 USA; 2012. [Google Scholar]
- 16. Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821–2830. [DOI] [PubMed] [Google Scholar]
- 17. Harel Z, Sholzberg M, Shah PS, et al. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol. 2014;25(3):431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boonyawat K, Caron F, Li A, et al. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(7):1322–1333. [DOI] [PubMed] [Google Scholar]
- 19. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryk AH, Pirog M, Plens K, et al. Heavy menstrual bleeding in women treated with rivaroxaban and vitamin K antagonists and the risk of recurrent venous thromboembolism. Vascul Pharmacol. 2016;87:242–247. [DOI] [PubMed] [Google Scholar]
- 21. Myers B, Webster A. Heavy menstrual bleeding on rivaroxaban – comparison with apixaban. Br J Haematol. 2017;176(5):833–835. [DOI] [PubMed] [Google Scholar]
- 22. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. [DOI] [PubMed] [Google Scholar]
- 23. Malec K, Goralczyk T, Undas A. The use of direct oral anticoagulants in 56 patients with antiphospholipid syndrome. Thromb Res. 2017;152:93–97. [DOI] [PubMed] [Google Scholar]