Abstract
New oral anticoagulants (NOACs; ie, direct thrombin inhibitor [DTI] and factor Xa [FXa] inhibitors) were used as alternatives to warfarin. Specific antidotes (idarucizumab for dabigatran and andexanet alfa for FXa inhibitors) and hemostatic reversal agents were used for lowering bleeding, but their efficacies were still uncertain. The objectives of this study were to estimate and compare the efficacy of NOAC antidotes on bleeding reversal and death. Studies were identified from MEDLINE and Scopus databases until May 2018. Case reports/series and cohorts were selected if they assessed reversal or death rates. Data were independently extracted by 2 reviewers. Individual patient data and aggregated data of outcomes were extracted from case reports/series and cohorts. Binary regression was used to estimate outcome rates, risk ratio (RR) along with 95% confidence interval (CI). Interventions were NOACs and reversal agents (ie, DTI-specific, DTI-standard, FXa-specific, and FXa-standard). Among 220 patients of 93 case reports/series, reversal rates were 95.9%, 77.6%, and 71.5% for DTI-specific, FXa-standard, and DTI-standard. Pooled RRs for DTI-specific and FXa-standard versus DTI-standard, respectively, were 1.34 (CI: 1.13-1.60) and 1.09 (CI: 0.84-1.40). Death rate was 0.18 (CI: 0.06-0.57) times lower in DTI-specific versus DTI-standard. For pooling 10 subcohorts, pooled RRs were 1.08 (CI: 1.00-1.16), 1.29 (CI: 1.20-1.39), and 1.13 (CI: 1.01-1.25) for DTI-specific, FXa-specific, and FXa-standard versus DTI-standard. In conclusion, specific reversal agents might be useful for reversal of bleeding and lowering the risk of death than standard reversal agents. Our findings were based on case reports/series and selected cohorts, further comparative studies are thus needed.
Keywords: NOAC, antidotes, reversal agents, bleeding reversal, stroke
Introduction
Warfarin is a commonly used anticoagulant worldwide for the prevention of thrombotic events.1 It is also used in nonvalvular atrial fibrillation (AF) for stroke prevention and the treatment of venous thromboembolism (VTE), for example, deep vein thrombosis and pulmonary embolism.2 The advantages of warfarin include convenience (once daily dosing), ability for monitoring (international normalized ratio [INR]) and specific antidote (vitamin K) for bleeding events.
However, many challenges and precautions should be considered during warfarin treatment. Firstly, warfarin interacts with other medications (ie, antifungals, antibiotics, and lipid lowering agents), alcohol, and food (ie, green vegetables, ginkgo, garlic, etc). Secondly, it causes severe hemorrhage due to its narrow therapeutic range. Thirdly, it requires frequent INR monitoring for dose adjustment, so patients may have inadequate anticoagulation if INR is too low or from bleeding if the INR is too high. In addition, dose adjustments can complicate prescribing and cause medication errors and poor adherence. Finally, initiating warfarin requires the concurrent use of other parenteral anticoagulants (eg, enoxaparin) to achieve warfarin optimal level.
New oral anticoagulants (NOACs), direct thrombin inhibitor (DTI), and factor Xa (FXa) inhibitor have recently been developed. Four NOACs are currently prescribed (ie, dabigatran [Pradaxa®, Boehringer Ingelheim Pharmaceuticals Inc., Germany], rivaroxaban [Xarelto®, Janssen Pharmaceuticals Co., Belgium], apixaban [Eliquis®, Bristol-Myers Squibb Co., USA], and edoxaban [Lixiana®/Savaysa®, Daiichi Sankyo Co., Japan]) for the treatment of VTE3 and prevention of stroke in patients with AF.2,4–6 Betrixaban (Bevyxxa), another FXa inhibitor was recently approved in June 2017 for VTE prophylaxis. Factor Xa inhibitors are also indicated for thromboprophylaxis in orthopedic surgery.7–9 Many advantages of these drugs are claimed over warfarin including less drug interactions, rapid onset of action without initial parenteral anticoagulation, fixed once or twice daily dose without the need for frequent monitoring or dose adjustment. These advantages can significantly simplify treatment and improve patients’adherence.10
Previous evidence showed that NOACs were noninferior, and even superior in some cases to warfarin in stroke prevention and systemic embolism.2,4–6,11 with lower risk of major bleeding5 including intracranial hemorrhage (ICH),2,4–6,12 and similar risk of major gastrointestinal bleeding.2,4,5,10
Despite their short action, reversal agents are needed to reverse anticoagulation effects in major bleeding or critical care practice. Hemostatic agents have been used for this purpose due to the absence of specific antidotes. Preclinical studies were conducted to assess the efficacy of hemostatic agents including coagulation factor replacement13–18 including prothrombin complex concentrate (PCC), activated PCC (aPCC; ie, FEIBA®, Shire US Inc., UK/USA; Cofact®, Sanquin, The Netherlands) and recombinant factor VIIa (rFVIIa, Novoseven®, Novo Nordisk, Denmark). Results only demonstrated the ability to normalize the prolonged prothrombin time (PT) caused by rivaroxaban but could not reverse the prolonged activated partial thromboplastin time caused by dabigatran.13
The United States Food and Drug Administration (US FDA) had approved idarucizumab (Praxbind®, Boehringer Ingelheim Pharmaceuticals Inc., Germany) in 2015 for the reversal of dabigatran. Idarucizumab is a monoclonal antibody fragment that binds free and thrombin-bound dabigatran and reverses its activity.19 However, conventional hemostatic agents are still important because of less accessibility of idarucizumab due to its high cost. The FDA approval was based on preclinical studies in healthy volunteers and cohort studies, but good evidence of its efficacy is still limited.20–25 Furthermore, idarucizumab is only specific to dabigatran, so the use of hemostatic agents is still crucial. Although there are specific antidotes for FXa inhibitors (ie, andexanet alfa and ciraparantag), clinical use and data are still limited because andexanet alfa has just recently been approved by the US FDA in May 2018, whereas ciraparantag is still waiting for the FDA approval.26–28 Therefore, conventional nonspecific hemostatic agents are still routinely prescribed.
A previous systematic review29 compared the outcomes between PCC, aPCC, and rFVIIa for the reversal of NOACs in which data were mainly from animal studies with 2 healthy volunteers without bleeding studies,13,14 but no definite conclusions were provided due to the lack of data in human studies. Another systematic review and meta-analysis30 was later conducted addressing the efficacy and safety of reversal of NOACs including 8 volunteer studies. Coagulation parameters were pooled including PT and endogenous thrombin potential.
Other human studies have recently been published, but they were limited to serum samples or randomized crossover trials in healthy volunteers without underlying thrombotic risks or clinical bleeding.13,16–18,22,23,31 There were a number of case reports, case series, and cohorts in which patients with NOAC-related bleeding were small subgroups of those cohorts. Additionally, the studies reported reversal agents for descriptive purposes rather than assessing treatment effectiveness.24,32–44 Therefore, we conducted a systematic review and meta-analysis to explore all regimens used and their efficacy in reversing the effect of NOACs and mortality using data from these kinds of studies.
Methods
The systematic review protocol was developed according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) statement45 and registered at PROSPERO which is an intermational database of prospectively registered systematic reviews (registration number: CRD42016032983).
Search Strategy and Selection Criteria
Studies published in English were identified from MEDLINE via PubMed and Scopus databases until May 3, 2018. Search terms were constructed mainly based only on interventions to yield more relevant studies including NOACs and antidotes as follows: NOAC (eg, direct oral anticoagulant), anticoagulant classes (ie, DTI and FXa inhibitor), and their product names. Antidotes included PCCs, activated PCCs, rFVIIa, and fresh frozen plasma (FFP), and idarucizumab (Online Appendix A).
Eligibility Criteria
Studies were included if they were descriptive or comparative observational studies or experimental designs conducted in humans; patients experienced bleeding from NOACs for stroke prevention in AF or VTE treatment; used at least one of the following antidote/reversal agents: PCC, aPCC, rFVIIa, FFP, or specific antidotes (idarucizumab or andexanet alfa); and reported bleeding reversal or in-hospital death or death within 30 days. Studies were excluded if patients received NOACs for VTE prophylaxis in orthopedic surgery or if data were insufficient.
Study Selection and Data Extraction
Selection of studies was done by 2 independent reviewers (S.U. and S.R.). Full texts were retrieved for review if a decision could not be made from the title and abstract. Studies were selected according to eligibility criteria. Disagreement of selections was resolved by consensus with a third party (A.T.).
Three reviewers (S.U., S.R., and T.A.) independently extracted data. For cohorts, aggregated data of treatments and outcomes were extracted. For case report/series, patients’ characteristics, interventions, and outcome data for individual patients (IPD) were extracted. Any disagreement was resolved by discussion/consensus with a third party (A.T.).
Interventions
New oral anticoagulants were classified as DTI (dabigatran) and FXa inhibitors (rivaroxaban, apixaban, and edoxaban). Reversal agents were also classified into standard (ie, PCC, aPCC, rFVIIa, and FFP) and specific groups (ie, idarucizumab for dabigatran, andexanet alfa for FXa inhibitors). Patients who received idarucizumab or andexanet alfa as monotherapy or combined with hemostatic agents were classified as specific group, otherwise were classified as standard reversal agent group. Combined NOACs and reversal agents were further classified as 4 treatment regimens of interest including dabigatran-idarucizumab (DTI-specific), dabigatran-standard reversal agents (DTI-standard), FXa inhibitors-andexanet alfa (FXa-specific), and FXa inhibitors-standard reversal agents (FXa-standard).
Outcomes
The outcomes of interest were reversal of bleeding and deaths. Bleeding reversal was defined according to the original studies including bleeding cessation, no hematoma expansion by computerized tomography (CT), clinically stable, normalized coagulation parameters, and survived or discharged.
Statistical Analysis
For cohort or subcohort studies, aggregated data were expanded to IPD data. For case reports/series, patients’ characteristics were compared between interventions and/or outcomes using t test for continuous data and χ2 or Fisher exact test where appropriate for categorical data. The reversal and death rates along with their 95% confidence intervals (CIs) were calculated by the type of interventions. Binary regression was applied to compare bleeding reversal between DTI-specific, DTI-standard, FXa-specific, and FXa-standard. A risk ratio (RR) along with its 95% CI was then estimated. All analyses were performed using STATA software version 14. Two-sided P < .05 was considered statistically significant.
Results
Study Selection
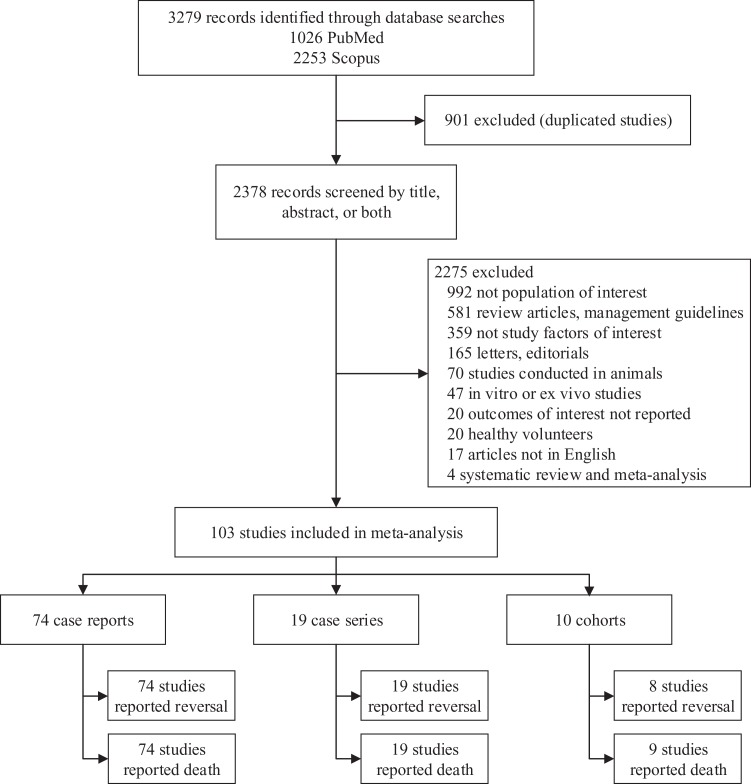
A total of 1026 and 2253 relevant studies were respectively identified from MEDLINE and Scopus databases, 901 duplicate studies were removed leaving 2378 studies for screening titles/abstracts. Among them, 2275 studies were later excluded with provided reasons leaving 103 studies for eligibility. Among them, 74, 19, and 10 were case reports, case series, and subcohort studies, respectively (Figure 1).
Figure 1.
Study flow from literature search.
Case Reports/Case Series
Characteristics of included studies and participants
Among 93 case reports/series, most studies were from the United States (54.8%). The number of included patients was 85 and 135 for case reports and series, respectively, with a total of 220 patients. The characteristics of these patients were summarized in Table 1. A total of 120 (61.9%) were male, and mean age was 77.3 years (standard deviation [SD] = 9.6). Among 152 patients with medical history, hypertension was the majority (50.7%), followed by coronary artery disease (20.4%), renal impairment (17.8%), diabetes (15.1%), and cerebrovascular disease (13.8%).
Table 1.
Characteristics of Included Participants From Case Reports/Case Series.a
| Characteristics | n | n (%) |
|---|---|---|
| Number of patients, n (%) | 220 | |
| Case report | 85 (38.64) | |
| Case series | 135 (61.36) | |
| Sex, n (%) | 194 | |
| Male | 120 (61.86) | |
| Female | 74 (38.14) | |
| Age, years, mean (SD) | 213 | 77.34 (9.55) |
| Medical history, n (%) | ||
| Hypertension | 152 | 77 (50.66) |
| Coronary artery disease | 152 | 31 (20.39) |
| Renal impairment | 152 | 27 (17.76) |
| Diabetes | 152 | 23 (15.13) |
| Cerebro vascular disease | 152 | 21 (13.82) |
| Renal function at baseline | ||
| SCr, mg/dL, mean (SD) | 11 | 1.20 (0.30) |
| CrCl, mL/min, mean (SD) | 8 | 47.58 (22.79) |
| Renal function on admission | ||
| SCr, mg/dL, median (range) | 41 | 1.75 (0.74, 11.27) |
| CrCl, mL/min, median (range) | 69 | 53 (14, 113) |
| Concomitant antiplatelet, n (%) | 90 | 36 (40.00) |
| Cause of bleeding, n (%) | 220 | |
| Spontaneous | 146 (66.36) | |
| Trauma | 64 (29.09) | |
| Postprocedure | 10 (4.55) | |
| Site of bleeding, n (%) | 220 | |
| Intracranial | 129 (58.64) | |
| Gastrointestinal | 55 (25.00) | |
| Other | 36 (16.36) |
Abbreviations: SCr, serum creatinine; SD, standard deviation.
a n, total number of participants available for that data.
Mean serum creatinine (SCr) and creatinine clearance at admission were 1.2 mg/dL (SD = 0.3) and 47.6 mL/min (SD = 22.8), respectively. Of 90 patients with home medications, 40.0% took antiplatelet medications concomitantly. Most of the patients (146/220, [66.4%]) had spontaneous bleeding, 64 (29.1%) were trauma related, and 10 (4.6%) were bleeding from any medical procedure without receiving a reversal agent before the procedure. The most prevalent bleeding site was ICH (129/220 [58.6%]) followed by gastrointestinal bleeding (55/220 [25.0%]).
Descriptions of NOACs
Prevention of stroke in nonvalvular AF (85.5%) was the most common indication for NOACs. Dabigatran was the most commonly used in 136 (61.8%) of 220 patients, while rivaroxaban and apixaban were, respectively, used in 68 (30.9%) and 16 (7.3%) patients, and none used edoxaban or betrixaban. Doses per day could be calculated in 94/136, 31/68, and 9/16 patients who took dabigatran, rivaroxaban, and apixaban, respectively. The median time since last NOAC dose was 11.5 hours (range: 1-96 hours; Appendix B). As for NOACs’ classes, 136 (61.8%) and 84 (38.2%) patients received the DTI (ie, dabigatran) and FXa inhibitors (ie, rivaroxaban and apixaban), respectively.
Descriptions of reversal agents
More patients received a single reversal agent than combined agents, that is, PCC was the most frequently used (30.9%), followed by idarucizumab (20.9%), FFP (13.2%), aPCC (12.7%), and rFVIIa (5.9%), whereas only 16.4% received combined agents with/without idarucizumab. Idarucizumab had a fixed dose of 5 g. Each reversal agent could not be analyzed individually due to the small number of observations. Therefore, reversal agents were recategorized into standard and specific agents for further analysis. Patients who received idarucizumab (either alone or combined) were categorized as specific group (50/220, 22.7%) and those did not receive idarucizumab were categorized as standard reversal agent group (170/220, 77.3%). Additional treatments for bleeding were used, that is, packed red blood cells transfusion (67/204, 32.8%) with a median of 4 (range: 1, 32) units, hemodialysis (27/200, 13.5%).
Our interventions of interest were combinations of NOACs and reversal agents: 86 (39.1%), 50 (22.7%), and 84 (38.2%) were included for DTI-standard, DTI-specific, and FXa-standard groups, respectively. These intervention groups were used for the main analysis. None of patients received andexanet alfa; therefore, there were no data for FXa-specific intervention.
Pooling of reversal outcome
Reversal of bleeding was variously defined across studies: 86/220 (39.1%) bleeding cessation, 68 (30.9%) stable/no hematoma, 29 (13.2%) survived or discharged, 23 (10.5%) clinical stability, and 14 (6.4%) normalization of coagulation parameters (Appendix C). Among 220 patients, 174 patients had bleeding reversal with a rate of 79.09% (95% CI: 71.58%-87.39%).
Characteristics of the included patients were compared between reversal groups (Appendix D). Although they were not significantly different between reversal and nonreversal groups, patients in the reversal group had a higher proportion of hypertension (52.8% vs 40.7%), diabetes (17.6% vs 3.7%), and use of concomitant antiplatelet (43.2% vs 25.0%), but lower creatinine clearance (46.4 vs 55.5 mL/min).
Indications for NOAC prescriptions were significantly different between reversal and nonreversal groups, that is, 87.9% versus 69.1% for AF, respectively. Types of NOACs were not much different between the groups except for rivaroxaban (29.3% vs 37.0%). However, the mean dosage of dabigatran (261.4 vs 285.9 mg/d, P = .027) and apixaban (7.1 vs 10.0 mg/d, P = .030) were significantly lower in reversal than the nonreversal groups, whereas rivaroxaban dosage was higher but not significant (19.2 vs 15.8 mg/d, P = .086). The median time since last NOAC dosage was 12 hours for both 2 groups. Use of specific antidote was higher (17.5% vs 4.8%, P = .040), or in other words, the use of standard reversal agents was lower in reversal than nonreversal groups (82.6% vs 95.2%). The total dosage for PCC, the most commonly used standard reversal agents, was higher in reversal than nonreversal groups (2476.4 vs 2154.0 units, P = .417) but was not statistically significant.
Site of major organ bleeding was explored by the intervention groups, indicating the most prevalent site of major organ bleeding was ICH (58.6%) followed by the GI bleeding (25.0%) and other (16.4%; Appendix E). Bleeding site varied by the intervention groups, that is, ICH was the higher in FXa-standard (76.2%) and DTI-specific (60.0%) than DTI-standard groups (40.7%). Therefore, the site of bleeding was considered in further analyses.
A binary regression was applied by fitting the intervention groups (ie, DTI-standard, DTI-specific, and FXa-Standard) on reversal event with adjustment for site of major organ bleeding (Table 2). This indicated that the reversal rate was highest in DTI-specific, followed by FXa-standard, and DTI-standard, that is, 95.9%, 77.6%, and 71.5%, respectively. The estimated RRs were respectively 1.34 (95% CI: 1.13-1.60) and 1.09 (95% CI: 0.84-1.40) for DTI-specific and FXa-standard groups compared to DTI-standard group. This could be interpreted that patients who received DTI-specific and FXa-standard reversal agents had approximately 34% and 9% higher chance of reversal than patients who received DTI-standard reversal agents, respectively.
Table 2.
Incidence and Risk Ratios Adjusted for Bleeding Sites in Case Reports/Case Series.
| Treatment Regimen | IR/100 | 95% CI | RR | 95% CI | P Value |
|---|---|---|---|---|---|
| Reversal | |||||
| FXa-standard | 77.60 | 63.83-91.37 | 1.09 | 0.84-1.40 | .526 |
| DTI-specific | 95.93 | 90.13-100 | 1.34 | 1.13-1.60 | .001 |
| DTI-standard | 71.46 | 58.92-84.00 | 1 | ||
| Death | |||||
| FXa-standard | 20.73 | 12.45-29.01 | 0.64 | 0.33-1.22 | .174 |
| DTI-specific | 5.95 | 0-11.94 | 0.18 | 0.06-0.57 | .003 |
| DTI-standard | 32.64 | 16.94-48.33 | 1 |
Abbreviations: CI, confidence interval; DTI, direct thrombin inhibitor; FXa, factor Xa; IR, incidence rate; RR, risk ratio.
Pooling of death outcome
A total of 48/220 patients died with a rate of 21.8% (95% CI: 15.70-30.32). Cerebrovascular disease was higher (18.2% vs 12.6%, P = .403), but hypertension (33.3% vs 55.5%, P = .024) and diabetes (6.1% vs 17.7%, P = .100) were lower in those who died versus alive (Appendix F). In addition, median creatinine clearance was lower (35.6 vs 53.0 mL/min, P = .271), and the use of concomitant antiplatelet was higher (52.9% vs 37.0%; P = .227), as well as greater intracranial bleeding (65.9% vs 57.8%; P = .502) in death than those who were alive but these were not significant.
Although NOAC types (dabigatran, rivaroxaban, and apixaban) were not much different between the groups, the mean dabigatran dosage (280.0 vs 262.0 mg/d, P = .085) was higher in death than alive. However, the percentage of patients receiving a specific antidote was significantly lower in death than alive (4.6% vs 17.7%; P = .031). In other words, standard reversal agents were prescribed more often in those who died (95.5% vs 82.3%), with mean total PCC dosage of 3017.8 versus 2179.3 units (P = .011).
Death rate was lowest in the DTI-specific group (6.0%) followed by FXa-standard (20.7%) and DTI-standard was highest (32.6%). The RRs were respectively 0.18 (95% CI: 0.06-0.57) and 0.64 (95% CI: 0.33-1.22) for DTI-specific and FXa-standard versus DTI-standard (Table 2).
Cohort Studies
Ten cohorts27,33,34,46–52 met our inclusion criteria. Among them, 6 studies27,34,46–50 were subcohorts, whereas 4 studies33,48,51,52 were the whole cohorts of NOAC-related bleeding with a total of 559 patients (Table 3). Dabigatran was the most commonly used (321/559, 57.4%), while rivaroxaban and apixaban were used in 238 (42.6%) patients. Three hundred twenty-one (57.4%) and 238 (42.6%) patients were in DTI and FXa inhibitor groups. Idarucizumab was the most frequently used antidote in 301 (53.9%) patients, followed by PCC (26.8%), 4F-PCC (8.6%), and andexanet alfa (8.2%).
Table 3.
Characteristics of Cohort Studies.
| Author (year) | Setting | Study Population (n1/n2) | Type of NOAC | Type of Antidote | Outcome | Definition of Reversal |
|---|---|---|---|---|---|---|
| Bouget and Oger (2015)46 | France | Patients with bleeding related to NOAC (6/54) | Rivaroxaban | 4F-PCC | Death | |
| Smythe et al. (2015)47 | United States | Patients with bleeding related to dabigatran or warfarin (3/105) | Dabigatran | 4F-PCC | Death | |
| Sin et al. (2016)34 | United States | Patients who received 4F-PCC (11/93) | Rivaroxaban, Apixaban, Dabigatran | 4F-PCC | Reverse | INR < 1.5 |
| Connolly et al. (2016)27 | United States, United Kingdom, Canada | Patients with bleeding related to FXa and receive andexanet alfa (46/67) | Rivaroxaban Apixaban | Andexanet alfa | Reverse | Excellent or good hemostasis efficacy |
| Pollack et al. (2017)49 | 38 countries | Patients with bleeding related to dabigatran and receive idarucizumab (301/503) | Dabigatran | Idarucizumab | Death, reverse | Stop bleeding in 24 hours |
| Schulman et al. (2017)33 | Canada | Patients with bleeding related to dabigatran (14/14) | Dabigatran | aPCC | Death, reverse | Effectiveness assessment for major bleeding management |
| Majeed et al. (2017)48 | Sweden | Patients with major bleeding related to rivaroxaban or apixaban with PCC (84/84) | Rivaroxaban, Apixaban | PCC | Death, reverse | Effectiveness assessment for bleeding management |
| Harrison et al. (2018)50 | United States | Patients with intracranial hemorrhage related to FXa versus vitamin K treated with 4F-PCC (14/42) | FXa inhibitor | 4F-PCC | Death, reverse | Hemorrhagic expansion |
| Schenk et al. (2018)51 | Austria | Patients with bleeding complications related to rivaroxaban (13/13) | Rivaroxaban | 4F-PCC | Death, reverse | Stop bleeding |
| Schulman et al. (2018)52 | Canada | Patients with major bleeding related to FXa treated with PCC (66/66) | Rivaroxaban Apixaban | PCC | Death, Reverse | Effectiveness assessment for major bleeding management |
Abbreviations: aPCC, activated prothrombin complex concentrate; FXa, factor Xa; INR, international normalized ratio; PCC, prothrombin complex concentrate.
a n1/n2: n1, number of patients included in our study, n2 number of patients in original cohort.
Pooling of reversal outcome
Eight of 10 cohorts reported bleeding reversal, 381/549 patients achieved bleeding reversal with an overall pooled reversal rate of 69.40% (95% CI: 65.36-73.23). Reversal rate was highest in FXa-specific, followed by FXa-standard, DTI-specific, and DTI-standard with pooled incidence rates of 80.43%, 75.57%, 67.44%, and 62.60%, respectively (Table 4). The incidence rate for each cohort study was presented in Appendix G. The estimated RRs for these 3 corresponding interventions versus DTI-standard were 1.29 (95% CI: 1.20-1.39), 1.13 (95% CI: 1.01-1.25), and 1.08 (95% CI: 1.00-1.16), respectively. This could be interpreted that patients who received FXa-specific, FXa-standard, and DTI-specific reversal agents had approximately 29%, 13%, and 8% higher bleeding reversal than patients who received DTI and standard reversal agents, respectively.
Table 4.
Incidence and Risk Ratios in Cohort Studies.
| Treatment Regimen | N/n | IR/100 | 95% CI | RR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Reversal | ||||||
| FXa-specific | 1/46 | 80.43 | 66.83-89.35 | 1.29 | 1.20-1.39 | < .001 |
| FXa-standard | 5/186 | 75.57 | 64.46-86.68 | 1.13 | 1.01- 1.25 | .030 |
| DTI-specific | 1/301 | 67.44 | 61.96-72.49 | 1.08 | 1.00-1.16 | .042 |
| DTI-standard | 2/16 | 62.60 | 39.00-86.20 | 1 | ||
| Death | ||||||
| FXa-specific | 1/46 | 15.22 | 7.57-28.22 | 0.91 | 0.25-3.37 | .891 |
| FXa-standard | 5/183 | 21.76 | 11.60-31.93 | 1.41 | 0.35-5.66 | .628 |
| DTI-specific | 1/301 | 6.31 | 4.08-9.65 | 0.38 | 0.10-1.40 | .145 |
| DTI-standard | 2/18 | 10.16 | 0.0-23.17 | 1 |
Abbreviations: CI, confidence interval; DTI, direct thrombin inhibitor; FXa, factor Xa; IR, incidence rate; N/n, numbers of included studies/numbers of patients; NOAC, new oral anticoagulant; RR, risk ratio.
Pooling of death outcome
Nine cohorts reported death outcome (n = 548) with a pooled death rate of 13.14% (95% CI: 10.42-16.26). Pooled death rate was lowest in DTI-specific, followed by DTI-standard, FXa-specific, and FXa-standard with pooled death rates of 6.31%, 10.16%, 15.22%, and 21.76%, respectively (Table 4). The death rate was estimated for each cohort (Appendix G) with the pooled RRs of 0.38 (95% CI: 0.10-1.40), 0.91 (95% CI: 0.25-3.37), and 1.41 (95% CI: 0.35-5.66) for DTI-specific, FXa-specific, and FXa-standard versus DTI-standard group. This could be interpreted that patients who received DTI-specific and FXa-specific antidotes were approximately 62% and 9% lower risk of death, respectively, while FXa-standard group had approximately 41% higher risk of death than those who received DTI-standard reversal agents.
Discussion
We conducted a systematic review and meta-analysis to assess the efficacy of reversal agents for different NOACs using evidence from 93 case reports/series (n = 220) and 10 cohorts (n = 559). Evidence from case reports/series suggested that 95.93%, 77.60%, and 71.46% of patients in the DTI-specific, FXa-standard, and DTI-standard groups, respectively, could achieve bleeding reversal. The DTI-specific and FXa-standard were 34% and 9% more likely to achieve bleeding reversal compared with DTI-standard group, respectively. In addition, the mortality was lowest in the DTI-specific group followed by the FXa-standard and DTI-standard. Results from cohorts were slightly different from case reports/series, that is, FXa-specific achieved highest bleeding reversal followed by FXa-standard and DTI-specific with 29%, 13%, and 8% higher than DTI-standard, although magnitudes of reversal rates were lower than those estimated from case reports/series. However, DTI-specific yielded lowest death rate compared to the rest.
Indications for NOAC use were mainly stroke prevention in AF (85.45%), followed by the treatment of VTE, and either AF or VTE (not specified). Both classes of NOACs (ie, DTI and FXa inhibitors) were approved for these 2 indications, but FXa inhibitors were approved only for VTE prophylaxis. We therefore did not consider including NOACs for VTE prophylaxis. Our study mainly focused on reversal outcomes, originally defined by the included studies, which mainly considered clinical signs and symptoms in addition to CT scanning. Although death was ascertained, specific cause of death was reported only in small numbers. In addition, we were also interested in other relevant surrogate outcomes (eg, INR, PT, activated partial thromboplastin, and thrombin time), but these surrogate outcomes were not commonly reported.
Our findings from case reports/series indicated that idarucizumab was promising in bleeding reversal and lowering the risk of death compared to standard reversal agents. This result was in accordance with a systematic review of dabigatran reversal using idarucizumab.53 A large prospective cohort also found that idarucizumab rapidly and safely reversed the anticoagulant effect of dabigatran.24,49 These studies highlighted the need for specific antidotes of NOACs in clinical practice.
We were able to assess the efficacy of FXa-specific group from 1 subcohort, which had quite small sample size (46) compared to sample size of its original cohort (67). Our findings suggested that 80.43%, 75.57%, 67.44%, and 62.60% of patients in the FXa-specific, FXa-standard, DTI-specific, and DTI-standard could achieve bleeding reversal, respectively. Patients in DTI-specific group had the lowest mortality rate (6.31%) when compared to DTI-standard groups (10.16%), FXa-specific (15.22%), and FXa-standard (21.76%). Our findings from cohort studies suggested that idarucizumab and andexanet alfa, NOAC-specific antidotes, were promising in bleeding reversal compared to standard reversal agents.
Idarucizumab might be the most effective for bleeding reversal and preventing death in patients taking dabigatran, whereas andexanet alfa might be the most effective for FXa inhibitors. Standard reversal agents for dabigatran and FXa inhibitors yielded lower efficacy in bleeding reversal than idarucizumab-dabigatran, but both were not much different. Therefore, where idarucizumab is not accessible, standard reversal agents might be the treatment of choice.
Physical approach using activated charcoal for reduction in anticoagulant absorption may be useful and had also been used in some cases.54,55 Given limited evidence from only one study,56 an activated charcoal was able to reduce apixaban exposure and facilitated its elimination when it was given up to 6 hours after apixaban overdose. A mechanical approach by dialysis might also be useful for clearance of serum level of dabigatran especially in cases with renal impairment.57–59
Dabigatran was the first-approved NOAC; therefore, there was evidence on dabigatran usage whereas evidence for other NOACs was limited. Although edoxaban and betrixaban had been approved by the US FDA since January 2015 and June 2017, respectively, they are still available in only some countries. Their data are still lack as for none of studies with edoxaban or betrixaban was identified by our search strategies. Thus, there is a big need for well-conducted studies evaluating the efficacy of specific antidotes.26,28 Before prescribing NOACs in clinical practice, physicians should account for baseline patients’ risks (eg, age, renal impairment, and site of bleeding [eg, ICH and GI bleeding])60,61 and availability of specific antidote in their settings.
Our study had some strengths. First, we included studies in which patients had bleeding in the real-world settings rather than using animal models or coagulopathy induction in healthy volunteers. Second, we could access IPD from 93 case reports/series with 220 patients, allowing us to explore patients’ characteristics and specific interventions which affected reversal and death outcomes. In addition, efficacy of interventions was assessed with adjustment for bleeding site.
However, there were some limitations. The best study design for assessing efficacy is randomized controlled trial or cohort. Although there were 10 cohorts which met our inclusion criteria, they were mainly small parts of original cohorts conducted for other purposes. In addition, these cohorts were only single-arm studies without comparing reversal or death with other interventions. Data were mainly from case reports/series in which interventions were not planned for direct comparisons. Therefore, selection bias might be present. Only patients who achieved bleeding reversal were selected and reported, whereas some other patients who failed to reverse might not have been reported. This hypothesis corresponded with results from pooling cohorts showing lower reversal rate in cohorts than case reports/series. Therefore, further comparative studies are needed to fill in the gap of knowledge. Publication bias is also likely because the patients represented here are likely to be more severe or at the “interesting” end of the spectrum, who were needed to break the publication barrier. Although we had attempted to pool treatment effects using cohort studies, they were mostly only minor parts of other cohorts which mainly aimed to assess other interests, not reversal rates in NOAC’s antidotes.
Conclusion
In summary, current evidence from case reports/series showed idarucizumab might be the most effective antidote for dabigatran-induced bleeding. Andexanet alfa is promising for bleeding reversal for FXa inhibitors. New oral anticoagulants should be used with caution considering the availability of specific antidote and patients’ risks. Further comparative studies particularly randomized controlled trials are needed.
Supplemental Material
Supplemental Material, Appendix_revise_11-06-2018 for The Reversal of Bleeding Caused by New Oral Anticoagulants (NOACs): A Systematic Review and Meta-Analysis by Sariya Udayachalerm, Sasivimol Rattanasiri, Teeranan Angkananard, John Attia, Nakarin Sansanayudh, and Ammarin Thakkinstian in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sasivimol Rattanasiri  http://orcid.org/0000-0001-7213-6116
http://orcid.org/0000-0001-7213-6116
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta-analysis. Circulation. 2015;132(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 3. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. [DOI] [PubMed] [Google Scholar]
- 7. Eriksson BI, Borris LC, Friedman RJ, et al. ; RECORD1 Study Group . Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. [DOI] [PubMed] [Google Scholar]
- 8. Lassen MR, Ageno W, Borris LC, et al. ; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776–2786. [DOI] [PubMed] [Google Scholar]
- 9. Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–2498. [DOI] [PubMed] [Google Scholar]
- 10. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. [DOI] [PubMed] [Google Scholar]
- 11. Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY Investigators. Oral Apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. [DOI] [PubMed] [Google Scholar]
- 12. Halperin JL, Hankey GJ, Wojdyla DM, et al. ; ROCKET AF Steering Committee and Investigators. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014;130(2):138–146. [DOI] [PubMed] [Google Scholar]
- 13. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. [DOI] [PubMed] [Google Scholar]
- 14. Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108(2):217–224. [DOI] [PubMed] [Google Scholar]
- 15. Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation. 2015;131(1):82–90. [DOI] [PubMed] [Google Scholar]
- 16. Levi M, Moore KT, Castillejos CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost. 2014;12(9):1428–1436. [DOI] [PubMed] [Google Scholar]
- 17. Cheung YW, Barco S, Hutten BA, Meijers JC, Middeldorp S, Coppens M. In vivo increase in thrombin generation by four-factor prothrombin complex concentrate in apixaban-treated healthy volunteers. J Thromb Haemost. 2015;13(10):1799–1805. [DOI] [PubMed] [Google Scholar]
- 18. Barco S, Whitney Cheung Y, Coppens M, Hutten BA, Meijers JC, Middeldorp S. In vivo reversal of the anticoagulant effect of rivaroxaban with four-factor prothrombin complex concentrate. Br J Haematol. 2016;172(2):255–261. [DOI] [PubMed] [Google Scholar]
- 19. Pakraftar S, Atencio D, English J, Corcos A, Altschuler EM, Stahlfeld K. Dabigatran etixilate and traumatic brain injury: evolving anticoagulants require evolving care plans. World J Clin Cases. 2014;2(8):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–1127. [DOI] [PubMed] [Google Scholar]
- 21. Schiele F, Van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121(18):3554–3562. [DOI] [PubMed] [Google Scholar]
- 22. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113(5):943–951. [DOI] [PubMed] [Google Scholar]
- 23. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386(9994):680–690. [DOI] [PubMed] [Google Scholar]
- 24. Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–520. [DOI] [PubMed] [Google Scholar]
- 25. Miyares MA, Kuyumjian Y, Eaves S, Dollard E. Idarucizumab, a humanized, monoclonal antibody fragment for immediate reversal of dabigatran. J Pharm Pract. 2015;28(6):548–554. [DOI] [PubMed] [Google Scholar]
- 26. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–2424. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Milling TJ, Eikelboom JW, et al. ; AMPLIFY Investigators. Andexanet alfa for acute major bleeding associated with Factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ansell JE, Bakhru SH, Laulicht BE, et al. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee FM, Chan AK, Lau KK, Chan HH. Reversal of new, factor-specific oral anticoagulants by rFVIIa, prothrombin complex concentrate and activated prothrombin complex concentrate: a review of animal and human studies. Thromb Res. 2014;133(5):705–713. [DOI] [PubMed] [Google Scholar]
- 30. da Luz LT, Marchand M, Nascimento B, Tien H, Nathens A, Shah P. Efficacy and safety of the drugs used to reverse direct oral anticoagulants: a systematic review and meta-analysis. Transfusion. 2017;57(7):1834–1846. [DOI] [PubMed] [Google Scholar]
- 31. Brown KS, Wickremasingha P, Parasrampuria DA, et al. The impact of a three-factor prothrombin complex concentrate on the anticoagulatory effects of the factor Xa inhibitor edoxaban. Thromb Res. 2015;136(4):825–831. [DOI] [PubMed] [Google Scholar]
- 32. Milling TJ, Fromm C, Ganetsky M, Pallin DJ, Cong J, Singer AJ. Management of major bleeding events in patients treated with dabigatran for nonvalvular atrial fibrillation: a retrospective, multicenter review. Ann Emerg Med. 2017;69(5):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schulman S, Ritchie B, Nahirniak S, et al. ; Study investigators . Reversal of dabigatran-associated major bleeding with activated prothrombin concentrate: a prospective cohort study. Thromb Res. 2017;152:44–48. [DOI] [PubMed] [Google Scholar]
- 34. Sin JH, Berger K, Lesch CA. Four-factor prothrombin complex concentrate for life-threatening bleeds or emergent surgery: a retrospective evaluation. J Crit Care. 2016;36:166–172. [DOI] [PubMed] [Google Scholar]
- 35. Beynon C, Sakowitz OW, Storzinger D, et al. Intracranial haemorrhage in patients treated with direct oral anticoagulants. Thromb Res. 2015;136(3):560–565. [DOI] [PubMed] [Google Scholar]
- 36. Purrucker JC, Haas K, Rizos T, et al. Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol. 2016;73(2):169–177. [DOI] [PubMed] [Google Scholar]
- 37. Albaladejo P, Samama CM, Sie P, et al. ; GIHP-NACO Study Group. Management of severe bleeding in patients treated with direct oral anticoagulants: an observational registry analysis. Anesthesiology. 2017;127(1):111–120. [DOI] [PubMed] [Google Scholar]
- 38. Hedges A, Coons JC, Saul M, Smith RE. Clinical effectiveness and safety outcomes associated with prothrombin complex concentrates. J Thromb Thrombolysis. 2016;42(1):6–10. [DOI] [PubMed] [Google Scholar]
- 39. Piccini JP, Garg J, Patel MR, et al. ; ROCKET AF Investigators. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J. 2014;35(28):1873–1880. [DOI] [PubMed] [Google Scholar]
- 40. Becattini C, Franco L, Beyer-Westendorf J, et al. Major bleeding with vitamin K antagonists or direct oral anticoagulants in real-life. Int J Cardiol. 2017;227:261–266. [DOI] [PubMed] [Google Scholar]
- 41. Dezman ZDW, Comer AC, Smith GS, Narayan M, Hess JR, Hirshon JM. The severity of bleeding and mortality in trauma patients taking dabigatran. J Emerg Med. 2016;51(3):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singer AJ, Quinn A, Dasgupta N, Thode HC. Management and outcomes of bleeding events in patients in the emergency department taking warfarin or a non–vitamin k antagonist oral anticoagulant. J Emerg Med. 2017;52(1):1–7.e1. [DOI] [PubMed] [Google Scholar]
- 43. Xu Y, Schulman S, Dowlatshahi D, et al. ; Bleeding Effected by Direct Oral Anticoagulants (BLED-AC) Study Group. Direct oral anticoagulant- or warfarin-related major bleeding: characteristics, reversal strategies and outcomes from a multi-center observational study. Chest. 2017;152(1):81–91. [DOI] [PubMed] [Google Scholar]
- 44. Sholzberg M, Pavenski K, Shehata N, Cserti-Gazdewich C, Lin Y. Bleeding complications from the direct oral anticoagulants. BMC hematology. 2015;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouget J, Oger E. Emergency admissions for major haemorrhage associated with direct oral anticoagulants. Thromb Res. 2015;136(6):1190–1194. [DOI] [PubMed] [Google Scholar]
- 47. Smythe MA, Forman MJ, Bertran EA, Hoffman JL, Priziola JL, Koerber JM. Dabigatran versus warfarin major bleeding in practice: an observational comparison of patient characteristics, management and outcomes in atrial fibrillation patients. J Thromb Thrombolysis. 2015;40(3):280–287. [DOI] [PubMed] [Google Scholar]
- 48. Majeed A, Agren A, Holmstrom M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130(15):1706–1712. [DOI] [PubMed] [Google Scholar]
- 49. Pollack CV, Jr., Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377(5):431–441. [DOI] [PubMed] [Google Scholar]
- 50. Harrison SK, Garrett JS, Kohman KN, Kline JA. Comparison of outcomes in patients with intracranial hemorrhage on factor Xa inhibitors versus vitamin K antagonists treated with 4-factor prothrombin complex concentrate. Proc (Bayl Univ Med Cent). 2018;31(2):153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schenk B, Goerke S, Beer R, Helbok R, Fries D, Bachler M. Four-factor prothrombin complex concentrate improves thrombin generation and prothrombin time in patients with bleeding complications related to rivaroxaban: a single-center pilot trial. Thromb J. 2018;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118(5):842–851. [DOI] [PubMed] [Google Scholar]
- 53. Thibault N, Morrill AM, Willett KC. Idarucizumab for reversing dabigatran-induced anticoagulation: a systematic review. Am J Ther. 2018;25(3):e333–e338. [DOI] [PubMed] [Google Scholar]
- 54. Peetermans M, Pollack C, Jr, Reilly P, et al. ; Study Investigators. Idarucizumab for dabigatran overdose. Clin Toxicol (Phila). 2016;54(8):644–646. [DOI] [PubMed] [Google Scholar]
- 55. Tummala R, Kavtaradze A, Gupta A, Ghosh RK. Specific antidotes against direct oral anticoagulants: a comprehensive review of clinical trials data. Int J Cardiol. 2016;214:292–298. [DOI] [PubMed] [Google Scholar]
- 56. Wang X, Mondal S, Wang J, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14(2):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol. 2013;8(9):1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790–1798. [DOI] [PubMed] [Google Scholar]
- 59. Marino KK, Santiago RA, Dew RB, 3 rd, et al. Management of dabigatran-associated bleeding with two doses of idarucizumab plus hemodialysis. Pharmacotherapy. 2016;36(10): e160–e165. [DOI] [PubMed] [Google Scholar]
- 60. Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol. 2015;262(3):516–522. [DOI] [PubMed] [Google Scholar]
- 61. Skaistis J, Tagami T. Risk of fatal bleeding in episodes of major bleeding with new oral anticoagulants and vitamin k antagonists: a systematic review and meta-analysis. Plos One. 2015;10(9):e0137444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Appendix_revise_11-06-2018 for The Reversal of Bleeding Caused by New Oral Anticoagulants (NOACs): A Systematic Review and Meta-Analysis by Sariya Udayachalerm, Sasivimol Rattanasiri, Teeranan Angkananard, John Attia, Nakarin Sansanayudh, and Ammarin Thakkinstian in Clinical and Applied Thrombosis/Hemostasis