Abstract
Background
Because of the increasing dysplasia rate in the lifelong course of inflammatory bowel disease (IBD) patients, it is imperative to characterize the crosstalk between IBD and colorectal cancer (CRC). However, there have been no reports revealing the occurrence of the ceRNA network in IBD-related CRC.
Methods
In this study, we conducted gene expression profile studies of databases and performed an integrated analysis to detect the potential of lncRNA-miRNA-mRNA ceRNA in regulating disease transformation. R packages were used to screen differentially expressed mRNA, lncRNA and miRNA among CRC, IBD and normal tissue. The lncRNA-miRNA-mRNA network was constructed based on predicted miRNA-targeted lncRNAs and miRNA-targeted mRNAs. Functional analyses were then conducted to identify genes involved in the ceRNA network, and key lncRNAs were evaluated based on several clinical outcomes.
Results
A total of three lncRNAs, 15 miRNAs, and 138 mRNAs were identified as potential mediators in the pathophysiological processes of IBD-related CRC. Gene Ontology annotation enrichment analysis confirmed that the dysplasia process was strongly associated with immune response, response to lipopolysaccharide, and inflammatory response. Survival analysis showed that LINC01106 (HR = 1.7; p < 0.05) were strongly associated with overall survival of colorectal cancer patients. The current study identified a series of IBD-related mRNAs, miRNA, and lncRNAs, and highlighted the important role of ceRNAs in the pathogenesis of IBD-related CRC. Among them, the LINC01106-miRNA-mRNA axis was identified as vital targets for further research.
Keywords: Inflammatory bowel disease, Colorectal cancer, Integrated bioinformatics, ceRNA network
Introduction
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD), and is a chronic and incurable inflammatory disorder most frequently affecting the gastrointestinal tract. The main pathological changes of IBD involve intestinal fibrosis, abscesses, fistulas, and ulcers (Baumgart & Sandborn, 2012; Danese & Fiocchi, 2011). According to a report by Kappelman in 2007 in the United States, the prevalence of CD and UC in children <20 years of age was 43 and 28 per 100,000, respectively, while among adults the prevalence was 201 and 238 per 100,000, respectively (Kappelman et al., 2007). In Europe, the prevalence of IBD ranged from 3–7 cases for CD and 4–11 cases for UC per 100,000 (Vegh, Kurti & Lakatos, 2017). The prevalence has also been reported to vary widely in Asian countries (6.5–121 per 100,000) (Ng et al., 2013; Thia et al., 2008). The etiology and pathogenesis of IBD still remain unclear, and abnormal immune regulation, gut commensal microbes, gastrointestinal environmental factors, and genetic susceptibility are commonly cited as key factors (Khor, Gardet & Xavier, 2011). A recent study has also reported that IBD is still a global disease with an accelerating incidence in newly industrialized countries (Ng et al., 2018). Patients with IBD need a lifelong course of drugs, which results in immense financial and psychological burdens to patients and their families.
IBD is a significant risk factor for colorectal cancer (CRC). In 2001, by summarizing 116 studies of the relationships between IBD and CRC, Eaden, Abrams & Mayberry (2001) concluded that the overall prevalence of CRC was 3.7% in UC patients. Another meta-analysis reported the prevalence of UC-associated CRC as 0.85%, which was elevated to nearly 14% at 30 years in those with long-standing extensive colitis (Bopanna et al., 2017). Comparable findings have been demonstrated in CD and the reported incidence was 8% at 22 years. Similarly, the cumulative risk of CRC in CD was reported as 0.3%, 1.6%, and 2.4% at 5, 15, and 25 years after diagnosis (Shawki et al., 2018). Furthermore, IBD-associated CRC patients exhibit differences both in chemotherapeutic and clinical outcomes (Axelrad et al., 2017).
The competing endogenous RNA (ceRNA) hypothesis provides a better understanding of tumorigenesis (Salmena et al., 2011; Wee et al., 2012). LncRNAs contain miRNA-response elements, which function as ceRNAs, interacting with miRNAs to indirectly regulate mRNAs. The molecular pathogenesis of IBD-associated CRC has been widely reported in recent studies. Kameyama et al. (2018) reported similar genomic alterations between samples obtained from CRC and colitis-associated colorectal cancer, except for some distinct variations in the times and frequencies. Although an expanding number of miRNAs and lncRNAs have been shown to play a role in IBD-induced CRC, few of them reported comprehensive lncRNA-mRNA-miRNA ceRNA networks during disease development (Josse & Bours, 2016; Kanaan et al., 2012; Lewis et al., 2017; Svrcek et al., 2013; Zhu et al., 2016).
In the present study, we identified a potential ceRNA network and characterized the potential mechanism during IBD-related CRC using bioinformatic analysis. We examined differentially expressed genes (DEGs) among CRC (or IBD related neoplasia), IBD and normal tissue based on three previously published gene datasets, GSE10714, GSE4183 and GSE68306, available in the Gene Expression Omnibus (GEO) database. By taking full advantage of The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov), an authoritative dataset COAD was used to identify abnormally expressed mRNAs, lncRNAs, and miRNAs in CRC compared to normal tissue. Together with four datasets, the crosstalk network among aberrant expressions of lncRNA-miRNA-mRNA was constructed to predict candidate diagnostic biomarkers and therapeutic targets for IBD-induced CRC. Further functional analyses, including Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, protein-protein interaction (PPI) network, transcription factor (TF) enrichment, and survival analysis were performed to explore potentially important genes involved in the transformation from IBD to CRC.
Materials & Methods
Expression profile dataset
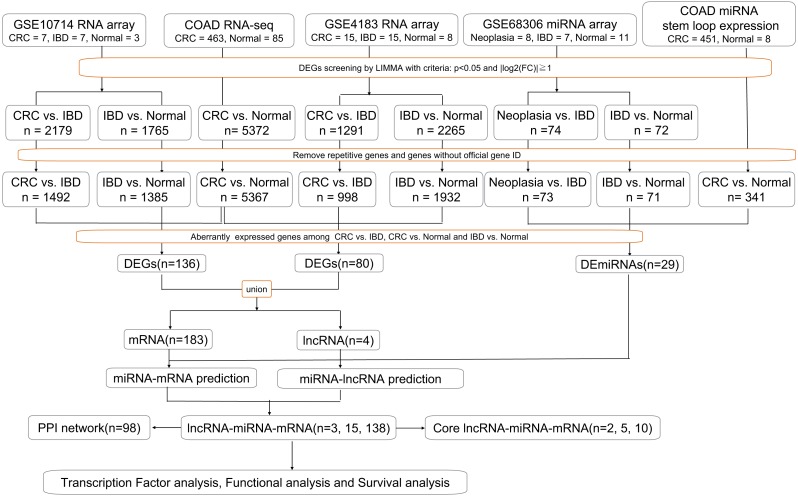
Gene expression profiles for IBD-related CRC (GSE10714, GSE4183 and GSE68306) were obtained from the GEO database. GSE10714 and GSE4183 were normalized genome-wide gene expression profiles, evaluated by HGU133 Plus 2.0 microarrays. In GSE4183, the RNA expression profiles of 15 patients with CRC, 15 with IBD, and eight healthy normal controls were included. In GSE10714, seven patients with CRC, seven with IBD (four with CD and three with UC), and three healthy normal controls were chosen. Meanwhile, GSE68306 is a miRNA array, in which samples were grouped into normal tissue (n = 16), UC without neoplasia (UC, n = 9), and UC-associated neoplastic tissue (n = 11). The quality of normalized gene expression data was analyzed and visualized using the ggplot2 package of R software for each group and sample. Moreover, COAD RNA-seq dataset was analyzed online by GEPIA based on TCGA database which contained 463 tumor tissue and 85 normal tissue (two recurrent and one metastasis tissue have been excluded). COAD miRNA expression profile containing 451 tumor sample and eight normal samples (one recurrent and one metastasis samples have been excluded) was obtained from XENA (a portal website for TCGA) and analyzed in R. All of the following processes have been shown in flow chart (Fig. 1).
Figure 1. Workflow of our bioinformatics analyses.
In the figure, “n” means number of genes.
Differential gene expression analyses
We analyzed the CRC/Neoplasia vs IBD groups and IBD vs Normal groups to identify the DEGs in GSE10714 and GSE4183, and differential expressed miRNAs (DE-miRNA) in GSE68306 by utilizing The Linear Models for Microarray data (LIMMA) package (Ritchie et al., 2015), which included lmFit, eBayes, and topTable functions. P < 0.05 and — log2-fold change —>1 were used as the cut-off criteria. The DEG screening between tumor tissue and normal tissue in COAD dataset was performed using the GEPIA (Tang et al., 2017) website with the same criteria by using LIMMA method, and their transcript biotypes were annotated using the bioMart package in R. DE-miRNAs in CRC vs Normal were screened by LIMMA in R with the same criteria as before based on COAD miRNA stem loop expression profile.
Constructing the ceRNA network
Along with identified differential expressed mRNAs, lncRNAs and miRNA, we constructed the ceRNA network as follows. 1. To predict the miRNA-targeted lncRNAs, we used DIANA-LncBase version 2 (http://carolina.imis.athena-innovation.gr/), starBase (http://starbase.sysu.edu.cn/) (Li et al., 2014; Yang et al., 2011), and RAID version 2.0 (http://www.rna-society.org/raid/) (Yi et al., 2016) to analyze interactions between lncRNAs and miRNAs. 2. To identify the miRNA-targeted mRNAs, we used DIANA-TarBase version 8 (http://carolina.imis.athena-innovation.gr/) and TargetScan (http://www.targetscan.org/). 3. To construct and visualize the LncRNA-miRNA-mRNA network, Cytoscape 3.7.0 software was used to construct and visualize the ceRNA network as well as its core network according to synergic expression of mRNA and lncRNA.
Functional analyses
GO annotation, including molecular function, cell component and biological process, and KEGG, summarizing genomes, biological pathways and health information, were used to clarify the potential role of the DEGs in the ceRNA network. The functional analysis was conducted using DAVID (Database for Annotation, Visualization and Integrated Discovery, version 6.8) (Huang, Sherman & Lempicki, 2008a; Huang, Sherman & Lempicki, 2008b), with P < 0.05 used as a cut off. The upstream transcription factors enriched for these DEGs were calculated using the UCSC Genome Browser (http://genome.ucsc.edu/). We constructed a PPI network for the DEGs using STRING (Szklarczyk et al., 2018), an online functional protein association network tool, to detect potential relationships among the DEGs with confidence scores ≥ 0.4 and a maximum number of interactors of one. The CentiScaPe plugin (Scardoni et al.) was used to determine the characteristics of each node in the PPI network, which assigned each gene a degree score, the simplest topological index, allowing for immediate evaluation of the average number of edges (interactions) incident to the node.
Survival analysis
Because lncRNAs listed in the ceRNA network can potentially regulate mRNA expression by sponging related miRNA, they are identified as pivotal genes in the network. LncRNAs correlations with stage expressions and patient survival were featured in GEPIA based on available TCGA patient survival data incuding COAD and READ datasets and Kaplan–Meier survival analysis is applied to generate overall and disease-free survival plots online.
Statistical analysis
Statistical analyses were performed using R studio. Expression Data were shown as mean ± SD. Paired sample t test, one-way ANOVA test, and the rank-sum test were flexibly used in different experiments. A 2-tailed P value less than 0.05 was considered to be statistical significant.
Results
Data distribution analyses and DEG screening
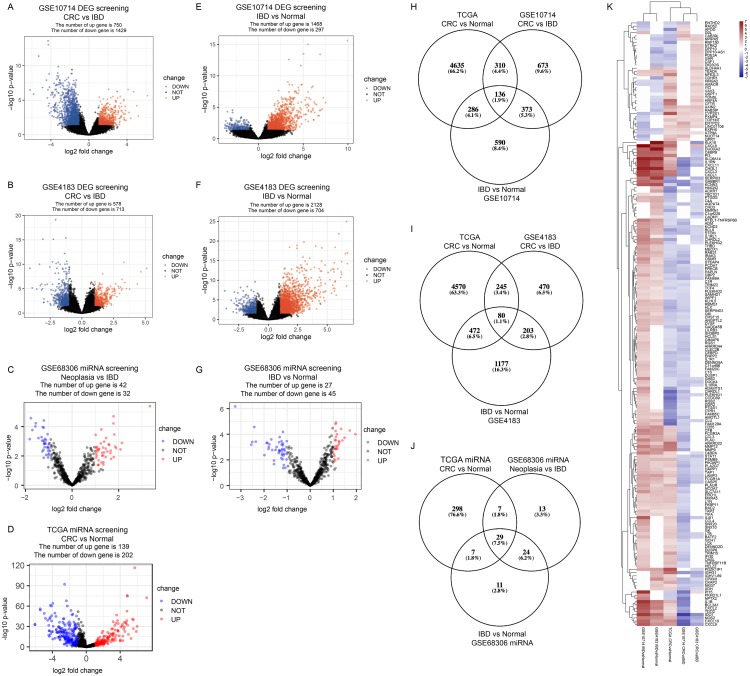
In GSE10714 and GSE4183, a total of 54,675 genes were detected in 17 samples. The expression values and the distributions were similar between the three groups in each dataset (Figs. S1A, and S1D for GSE10714; Figs. S1B, and S1E for GSE4183). These data distribution analyses showed that the two GEO datasets were qualified for further bioinformatics analyses. In GSE68306 miRNA array dataset, a total of 654 miRNAs were detected in 36 samples. Among them, samples Normal 12-16, IBD 8-9, and Neoplasia 9-11 were ruled out because data distribution of these samples were quite different from others in the same group (Fig. S1F). The expression density of three groups in GSE68306 is similar after filtration (Fig. S1C). Following data processing using LIMMA, we identified 2,179 DEGs, 1,291 DEGs, and 74 DE-miRNAs in the CRC/Neoplasia group vs. the IBD group for GSE10714 (Fig. 2A), GSE4183 (Fig. 2B) and GSE68306 (Fig. 2C), respectively; meanwhile, 1765 DEGs, 2265 DEGs and 72 DE-miRNAs were identified in the IBD group vs. the Normal group for GSE10714 (Fig. 2E), GSE4183 (Fig. 2F) and GSE68306 (Fig. 2G), respectively. According to GEPIA, 5,372 DEGs in the CRC group vs. the Normal group were obtained with the same cut-off criteria. Also, 341 DE-miRNAs in the CRC group vs. the Normal group were identified by R based on COAD miRNA expression profile (Fig. 2D). Repetitive genes with different probes and these predicted genes without official gene symbol or transcript annotation in NCBI or Ensembl were removed in the following analysis.
Figure 2. Identify differential expressed genes in the disease progression from IBD to CRC.
(A–G) Volcano graph displaying pairs of expressed genes. (H) Venn analysis among DEGs of CRC vs. IBD in GSE10714, IBD vs. Normal in GSE10714, GSE4183, and CRC vs Normal in TCGA. (I) Venn analysis among DEGs of CRC vs. IBD in GSE4183, IBD vs. Normal in GSE4183, and CRC vs. Normal in TCGA. (J) Venn analysis among DE-miRNAs of CRC vs. IBD in GSE68306, IBD vs. Normal in GSE68306, and CRC vs. Normal in TCGA miRNA. (K) Heatmap and hierarchical clustering of identified 187 DEGs. Up or down regulated genes are colored in red or blue, respectively.
Disease progression associated DEGs
In our study, we meant to compare the differences between IBD and CRC to figure out potential genomic change that contribute to the disease progression from IBD to CRC. Also, we need to make sure these DEGs are both significantly differential expressed in CRC vs. Normal comparison and IBD vs. Normal comparison which make sense that they are involved in the development of tumorigenesis. By intersection using three comparisons (CRC/Neoplasia vs. IBD, IBD vs. Normal and CRC vs Normal) and combining data in two intersections(Figs. 2H and 2I), we identified a total of 187 DEGs as the IBD-related DEGs, including four lncRNAs and 183 mRNAs. Hierarchical clustering of the identified DEGs is displayed as a heat map to show the gene clusters sharing similar expression change among CRC, IBD and normal tissue (Fig. 2K) which indicated that most of DEGs are higher expressed in IBD than CRC. By intersection using three comparisons of miRNA data, 29 miRNAs are recognized (Fig. 2J). The information for these DEGs, DE-miRNAs and heatmap expression data was shown in Tables S1–S9.
Construction of the ceRNA network
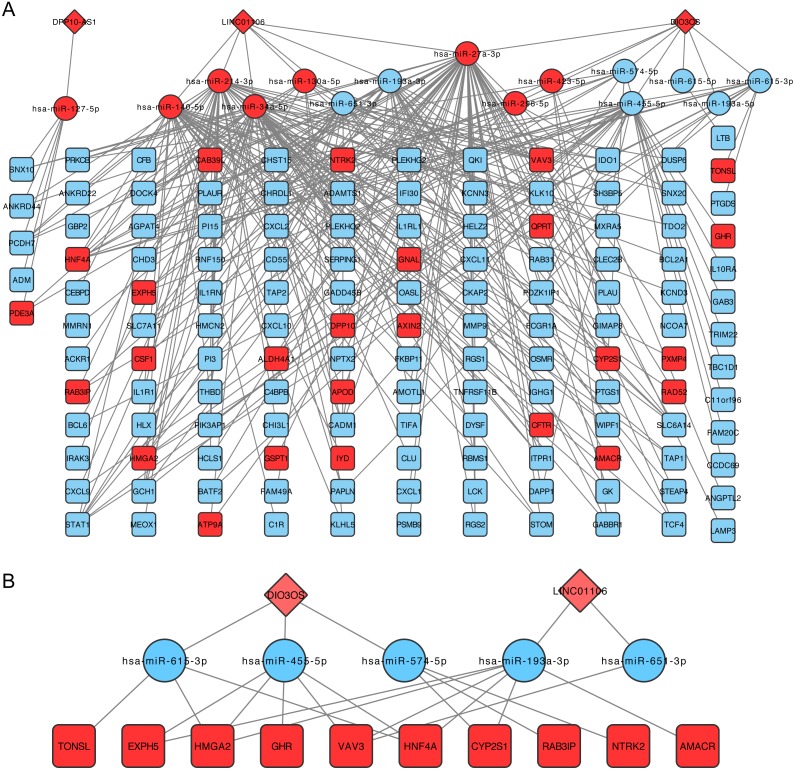
The lncRNA-miRNA-mRNA network was then constructed following the steps listed in the Materials and Methods, which contained three lncRNA, 15 miRNAs, and 138 mRNA (Fig. 3A). CeRNA is said to act as a sponge for miRNAs and free mRNA from miRNA binding. Therefore, a core ceRNA network was extracted including synergic expressed mRNA and lncRNA but divergent expressed miRNAs, and shown in Fig. 3B. These three lncRNAs in Fig. 3A were termed key ceRNAs. The interactions and nodes information in ceRNA network were shown in Tables S10 and S11, respectively.
Figure 3. lncRNA-miRNA-mRNA ceRNA network.
(A) is the total ceRNA network and (B) is the core ceRNA network containing synergic expressed mRNA and lncRNA but divergent expressed miRNAs. Diamonds stand for lncRNAs, rectangles stand for mRNAs, and ellipses stand for mRNA. Up or down regulated genes are colored in red or blue, respectively.
Functional analyses
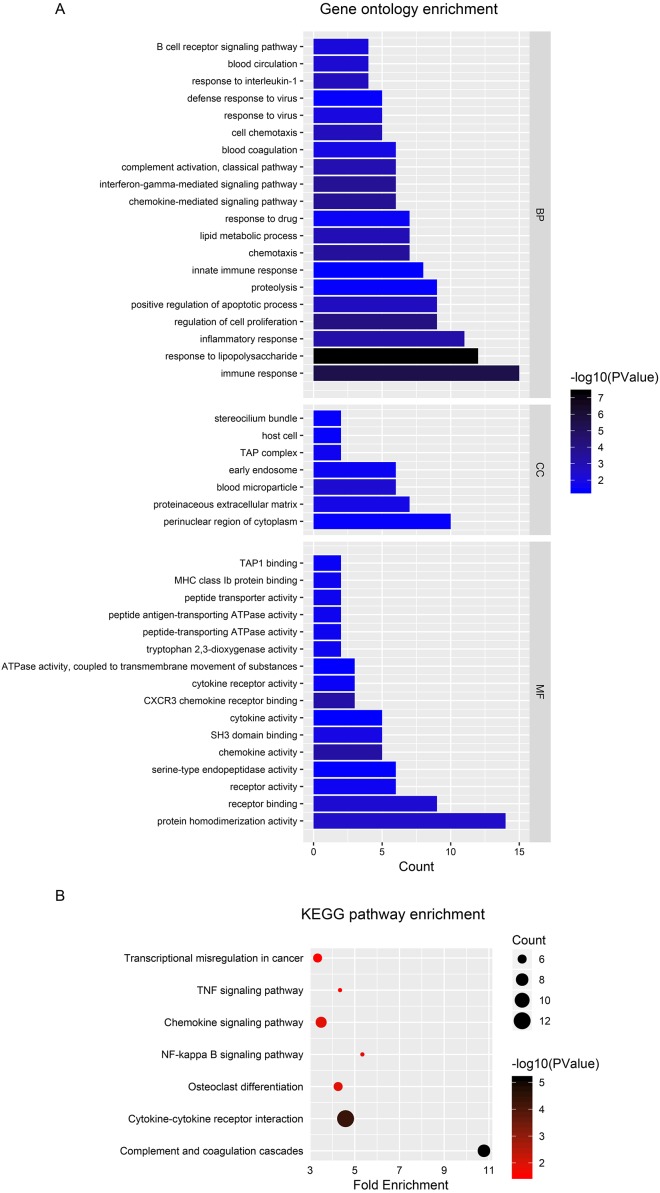
To explore the functional significance and potential regulators of the mRNAs involved in the ceRNA network, GO annotation and KEGG pathway enrichment analyses were conducted. Figure 4A shows that ceRNAs may affect the transformation from IBD to CRC by regulating the immune response, response to lipopolysaccharide and inflammatory response in biological processes. Significant cellular component terms revealed that proteins coded by these mRNAs were mainly located in the perinuclear region of cytoplasm. The top three molecular functions identified were protein homodimerization, receptor binding, and receptor activity. As for KEGG pathway enrichment, the ceRNA relevant mRNAs were mostly enriched in complement and coagulation cascades, cytokine-cytokine receptor interaction, and osteoclast differentiation (Fig. 4B). The detailed genes involved in each term are listed in the Tables 1 and 2.
Figure 4. Functional analysis of DEGs in ceRNA network.
(A) Histogram of Gene Ontology (GO) functional classification of DEGs. The x-axis represents the number of DEGs, with individual GO terms plotted on the y-axis. All GO terms were grouped into three categories: biological processes, cellular components, and molecular functions. The graph displays only significantly enriched GO terms (P < 0.05), with darker blue indicating greater significance. (B) Histogram of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment in DEGs. The x-axis represents the number of DEGs annotated in a pathway, with individual KEGG terms shown on the y-axis. The graph displays only significantly enriched KEGG terms (P < 0.05), with darker red indicating greater significance.
Table 1. Information of GO enrichment for DEGs.
| Category | Term | Count | P value | Genes |
|---|---|---|---|---|
| BP | Immune response | 15 | 3.68E-06 | CXCL1, IL1R1, IL1RL1, IL1RN, CXCL2, CXCL9, C1R, CXCL11, TRIM22, CXCL10, TNFRSF11B, RGS1, FCGR1A, LTB, GBP2 |
| BP | Response to lipopolysaccharide | 12 | 4.62E-08 | CXCL1, IRAK3, TNFRSF11B, THBD, ADM, IL10RA, CXCL2, CXCL9, IDO1, CXCL11, CXCL10, GCH1 |
| BP | Inflammatory response | 11 | 6.13E-04 | CXCL1, TNFRSF11B, CSF1, CXCL2, PTGS1, ACKR1, CHI3L1, CXCL9, BCL6, CXCL11, CXCL10 |
| BP | Regulation of cell proliferation | 9 | 8.19E-05 | TNFRSF11B, CXCL2, LCK, PTGS1, CXCL9, BCL6, CXCL11, PLAU, CXCL10 |
| BP | Positive regulation of apoptotic process | 9 | 2.02E-03 | PLEKHG2, VAV3, ADM, BCL2A1, CLU, BCL6, GADD45B, HMGA2, DUSP6 |
| BP | Proteolysis | 9 | 3.62E-02 | IGHG1, CFB, MMP9, DPP10, ADAMTS1, KLK10, PAPLN, C1R, PLAU |
| BP | Innate immune response | 8 | 4.50E-02 | IGHG1, CD55, CSF1, LCK, CLU, SERPING1, C4BPB, C1R |
| BP | Chemotaxis | 7 | 3.28E-04 | CXCL1, CXCL2, CXCL9, CXCL11, PLAU, CXCL10, PLAUR |
| BP | Lipid metabolic process | 7 | 1.24E-03 | APOD, HNF4A, PTGDS, PTGS1, CLU, IL1RN, PDE3A |
| BP | Response to drug | 7 | 2.81E-02 | TNFRSF11B, VAV3, APOD, LCK, PDE3A, STAT1, DUSP6 |
| BP | Chemokine-mediated signaling pathway | 6 | 2.00E-04 | CXCL1, CXCL2, ACKR1, CXCL9, CXCL11, CXCL10 |
| BP | Interferon-gamma-mediated signaling pathway | 6 | 2.00E-04 | OASL, FCGR1A, IFI30, STAT1, TRIM22, GBP2 |
| BP | Complement activation, classical pathway | 6 | 9.27E-04 | IGHG1, CD55, CLU, SERPING1, C4BPB, C1R |
| BP | Blood coagulation | 6 | 1.30E-02 | THBD, HNF4A, C4BPB, MMRN1, PLAU, PLAUR |
| BP | Cell chemotaxis | 5 | 1.48E-03 | CXCL1, CXCL2, CXCL9, DOCK4, CXCL10 |
| BP | Response to virus | 5 | 9.73E-03 | IRAK3, OASL, CLU, HMGA2, TRIM22 |
| BP | Defense response to virus | 5 | 3.67E-02 | OASL, CXCL9, STAT1, TRIM22, CXCL10 |
| BP | Response to interleukin-1 | 4 | 1.95E-03 | IRAK3, IL1R1, CHI3L1, GHR |
| BP | Blood circulation | 4 | 4.76E-03 | ADM, SERPING1, STAT1, CXCL10 |
| BP | B cell receptor signaling pathway | 4 | 7.91E-03 | IGHG1, VAV3, LCK, PRKCB |
| CC | Perinuclear region of cytoplasm | 10 | 3.68E-02 | STOM, LAMP3, APOD, PTGDS, CSF1, ATP9A, CLU, CHI3L1, STA1, GBP2 |
| CC | Proteinaceous extracellular matrix | 7 | 1.34E-02 | TNFRSF11B, IL1RL1, MMP9, PI3, CHI3L1, ADAMTS1, PAPLN |
| CC | Blood microparticle | 6 | 5.01E-03 | IGHG1, STOM, CFB, CLU, SERPING1, C1R |
| CC | Early endosome | 6 | 2.57E-02 | RAB31, LAMP3, DYSF, ATP9A, ACKR1, CFTR |
| CC | TAP complex | 2 | 2.16E-02 | TAP2, TAP1 |
| CC | Host cell | 2 | 3.57E-02 | TAP2, TAP1 |
| CC | Stereocilium bundle | 2 | 3.57E-02 | IDO1, DOCK4 |
| MF | Protein homodimerization activity | 14 | 3.17E-03 | CADM1, CEBPD, CSF1, BCL2A1, STAT1, GCH1, STOM, IRAK3, HNF4A, TAP1, NTRK2, QPRT, TCF4, GHR |
| MF | Receptor binding | 9 | 5.01E-03 | CXCL1, ADM, CADM1, HNF4A, AMACR, ANGPTL2, LTB, CXCL10, PLAUR |
| MF | Receptor activity | 6 | 2.34E-02 | TNFRSF11B, THBD, CADM1, IL10RA, ACKR1, PLAUR |
| MF | Serine-type endopeptidase activity | 6 | 4.24E-02 | IGHG1, CFB, MMP9, KLK10, C1R, PLAU |
| MF | Chemokine activity | 5 | 4.84E-04 | CXCL1, CXCL2, CXCL9, CXCL11, CXCL10 |
| MF | SH3 domain binding | 5 | 1.22E-02 | SH3BP5, HCLS1, QKI, WIPF1, DOCK4 |
| MF | Cytokine activity | 5 | 4.29E-02 | TNFRSF11B, CSF1, IL1RN, CXCL9, LTB |
| MF | CXCR3 chemokine receptor binding | 3 | 5.45E-04 | CXCL9, CXCL11, CXCL10 |
| MF | Cytokine receptor activity | 3 | 2.95E-02 | IL1RL1, OSMR, GHR |
| MF | ATPase activity, coupled to transmembrane movement of substances | 3 | 4.26E-02 | TAP2, TAP1, CFTR |
| MF | Tryptophan 2,3-dioxygenase activity | 2 | 2.22E-02 | TDO2, IDO1 |
| MF | Peptide-transporting ATPase activity | 2 | 2.22E-02 | TAP2, TAP1 |
| MF | Peptide antigen-transporting ATPase activity | 2 | 2.22E-02 | TAP2, TAP1 |
| MF | Peptide transporter activity | 2 | 2.22E-02 | TAP2, TAP1 |
| MF | MHC class Ib protein binding | 2 | 2.22E-02 | TAP2, TAP1 |
| MF | TAP1 binding | 2 | 2.95E-02 | TAP2, TAP1 |
Table 2. Information of KEGG pathway enrichment for DEGs.
| Number | Term | Count | Fold enrichment | P value | Genes |
|---|---|---|---|---|---|
| hsa04610 | Complement and coagulation cascades | 8 | 10.78 | 7.23E-06 | CD55, THBD, CFB, SERPING1, C4BPB, C1R, PLAU, PLAUR |
| hsa04060 | Cytokine-cytokine receptor interaction | 12 | 4.59 | 4.36E-05 | CXCL1, IL1R1, TNFRSF11B, OSMR, IL10RA, CSF1, CXCL2, CXCL9, CXCL11, LTB, GHR, CXCL10 |
| hsa04380 | Osteoclast differentiation | 6 | 4.26 | 1.24E-02 | IL1R1, TNFRSF11B, FCGR1A, CSF1, LCK, STAT1 |
| hsa04064 | NF-kappa B signaling pathway | 5 | 5.34 | 1.34E-02 | IL1R1, LCK, BCL2A1, LTB, PLAU |
| hsa04062 | Chemokine signaling pathway | 7 | 3.50 | 1.37E-02 | CXCL1, VAV3, CXCL2, CXCL9, CXCL11, STAT1, CXCL10 |
| hsa04668 | TNF signaling pathway | 5 | 4.34 | 2.65E-02 | CXCL1, CSF1, MMP9, CXCL2, CXCL10 |
| hsa05202 | Transcriptional misregulation in cancer | 6 | 3.34 | 3.17E-02 | FCGR1A, MMP9, BCL2A1, BCL6, HMGA2, PLAU |
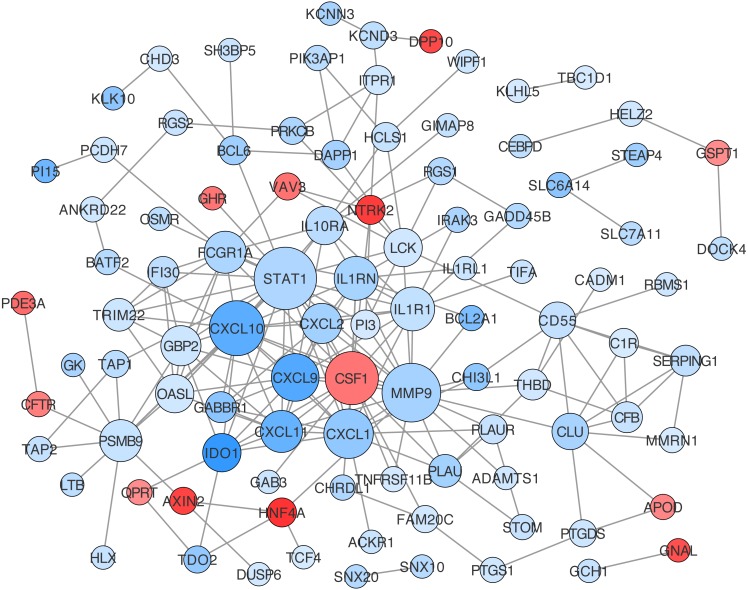
Construction of protein-protein interaction networks among these genes was conducted based on String in Cytoscape. The centrality degree of each node was evaluated by the CentiScape plugin. Twenty-six genes were defined as hub genes with the criterion, degree >5, including STAT1, MMP9, CXCL10, CSF1, CXCL1, CXCL9, IL1R1, IL1RN, CXCL11, PSMB9 and so on (Table S12). Among them, STAT1, MMP9, and CXCL10 were the top three nodes centralized in net with the largest size (Fig. 5).
Figure 5. Protein-protein interaction (PPI) network construction for DEGs in ceRNA network.
The value of centrality degree is marked by different node size. Up or down regulation of genes according to CRC vs. IBD comparison are filled with red or blue color, respectively.
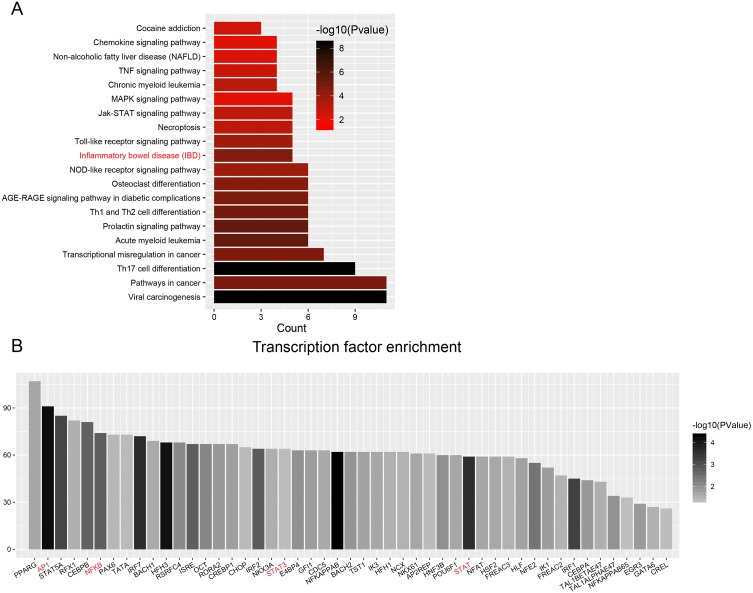
Enrichment analyses of transcription factors (TFs) of upstream DEGs in the ceRNA network indicated that inflammatory bowel disease (JUN, NFKB1, STAT1, STAT3 and GATA6) and pathways in cancer (CCDC6, CEBPA, JUN, NFKB1, NKX31, PPARG, RUNX1, STAT1, STAT3, STAT5A, and STAT5B) were included in the KEGG pathway list (Fig. 6A and Table 3). The number of DEGs in each TF is shown as a histogram in Fig. 6B. JUN (also known as AP1) and NFKB1 regulated more than 50% of the genes in the list. The DEGs targeted by TFs were listed in Table S13.
Figure 6. Transcription factor enrichment.
(A) KEGG pathway enrichment for enriched TFs. The graph displays only significantly enriched KEGG terms (P < 0.05), with darker red indicating greater significance. (B) Histogram of TFs shows the count of genes downstream each TF.
Table 3. KEGG pathway enrichment for transcription factors.
| KEGG Term | Count | P value | TFs |
|---|---|---|---|
| Viral carcinogenesis | 11 | 3.6E-09 | ATF2,EGR3,IRF3,IRF7,IRF9,JUN,NFKB1,STAT3,STAT5A,STAT5B,TBP |
| Pathways in cancer | 11 | 1.2E-05 | CCDC6,CEBPA,JUN,NFKB1,NKX3-1, PPARG, RUNX1, STAT1, STAT3, STAT5A, STAT5B |
| Th17 cell differentiation | 9 | 3.6E-09 | GATA3,IRF4,JUN,NFKB1,RUNX1,STAT1,STAT3,STAT5A,STAT5B |
| Transcriptional misregulation in cancer | 7 | 1.7E-05 | CEBPA,CEBPB,DDIT3,NFKB1,PPARG,RUNX1,ZEB1 |
| Acute myeloid leukemia | 6 | 2.9E-06 | CEBPA,NFKB1,RUNX1,STAT3,STAT5A,STAT5B |
| Prolactin signaling pathway | 6 | 3.1E-06 | IRF1,NFKB1,STAT1,STAT3,STAT5A,STAT5B |
| Th1 and Th2 cell differentiation | 6 | 8.8E-06 | GATA3,JUN,NFKB1,STAT1,STAT5A,STAT5B |
| AGE-RAGE signaling pathway in diabetic complications | 6 | 1.3E-05 | JUN,NFKB1,STAT1,STAT3,STAT5A,STAT5B |
| Osteoclast differentiation | 6 | 2.9E-05 | BLNK,IRF9,JUN,NFKB1,PPARG,STAT1 |
| NOD-like receptor signaling pathway | 6 | 1.3E-04 | IRF3,IRF7,IRF9,JUN,NFKB1,STAT1 |
| Inflammatory bowel disease (IBD) | 5 | 2.3E-05 | GATA3,JUN,NFKB1,STAT1,STAT3 |
| Toll-like receptor signaling pathway | 5 | 1.6E-04 | IRF3,IRF7,JUN,NFKB1,STAT1 |
| Necroptosis | 5 | 9.1E-04 | IRF9,STAT1,STAT3,STAT5A,STAT5B |
| Jak-STAT signaling pathway | 5 | 9.7E-04 | IRF9,STAT1,STAT3,STAT5A,STAT5B |
| MAPK signaling pathway | 5 | 9.2E-03 | ATF2,DDIT3,JUN,NF1,NFKB1 |
| Chronic myeloid leukemia | 4 | 7.4E-04 | NFKB1,RUNX1,STAT5A,STAT5B |
| TNF signaling pathway | 4 | 2.3E-03 | ATF2,CEBPB,JUN,NFKB1 |
| Non-alcoholic fatty liver disease (NAFLD) | 4 | 6.0E-03 | CEBPA,DDIT3,JUN,NFKB1 |
| Chemokine signaling pathway | 4 | 9.7E-03 | NFKB1,STAT1,STAT3,STAT5B |
| Cocaine addiction | 3 | 2.8E-03 | ATF2,JUN,NFKB1 |
| Cytosolic DNA-sensing pathway | 3 | 5.1E-03 | IRF3,IRF7,NFKB1 |
| Non-small cell lung cancer | 3 | 5.9E-03 | STAT3,STAT5A,STAT5B |
| RIG-I-like receptor signaling pathway | 3 | 6.5E-03 | IRF3,IRF7,NFKB1 |
| B cell receptor signaling pathway | 3 | 6.5E-03 | BLNK,JUN,NFKB1 |
| Pancreatic cancer | 3 | 6.9E-03 | NFKB1,STAT1,STAT3 |
| ErbB signaling pathway | 3 | 9.2E-03 | JUN,STAT5A,STAT5B |
| Longevity regulating pathway | 3 | 9.9E-03 | ATF2,NFKB1,PPARG |
| IL-17 signaling pathway | 3 | 1.1E-02 | CEBPB,JUN,NFKB1 |
| Prostate cancer | 3 | 1.2E-02 | NFKB1,NKX3-1,ZEB1 |
| Relaxin signaling pathway | 3 | 2.5E-02 | ATF2,JUN,NFKB1 |
| Fluid shear stress and atherosclerosis | 3 | 2.6E-02 | JUN,MEF2A,NFKB1 |
| Apoptosis | 3 | 2.6E-02 | DDIT3,JUN,NFKB1 |
| Maturity onset diabetes of the young | 2 | 1.1E-02 | FOXA2,PAX6 |
| Thyroid cancer | 2 | 1.9E-02 | CCDC6,PPARG |
| Amphetamine addiction | 2 | 4.6E-02 | ATF2,JUN |
| Adipocytokine signaling pathway | 2 | 5.0E-02 | NFKB1,STAT3 |
Survival analysis for ceRNA
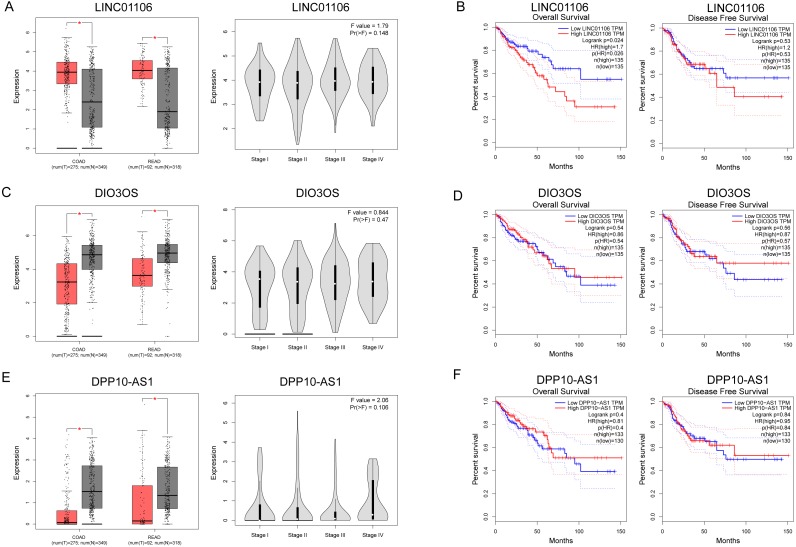
The expressions of three ceRNA from the TCGA dataset were plotted to reveal differences among Stages I–IV (Figs. 7A, 7C and 7E). None of them was significantly differently expressed in the higher stage (p > 0.05). Survival analysis showed that LINC01106 (Fig. 7B; HR = 1.7; p < 0.05) was strongly associated with overall survival of CRC patients.
Figure 7. Clinical significance of three key lncRNAs in ceRNA network.
(A, C & E) Analyses of key lncRNAs expression in tumor vs. normal tissues and in different tumor stages. Red box stands for tumor tissue; grey box is for normal tissue; and dots presents each sample value in left panel. (B, D & F) Overall and disease-free survival analyses of key lncRNAs. *P < 0.05; other P-values are shown on the diagrams. READ, rectum adenocarcinoma; COAD, colon adenocarcinoma.
Discussion
In the current study, we used bioinformatic analysis to identify IBD-related CRC mRNAs and lncRNAs based on the published database obtained from the GEO and TCGA. By constructing a lncRNA-miRNA-mRNA network, a total of three lncRNAs, 15 miRNAs, and 138 mRNAs were identified as potential mediators in the pathophysiological processes of IBD-related CRC. Moreover, we also performed functional analyses to investigate the potential biological functions and pathways involved in the disease progression from IBD to CRC.
According to our GO annotation results, DEGs included in ceRNA network, could regulate the IBD-related CRC through involvement in the regulation of the immune response, response to lipopolysaccharide (LPS), and inflammation. The immune response was shown to play an important role in the pathogenesis of IBD. LPS was well known as a TLR4 activator (Chowdhury, Sacks & Sheerin, 2006). As an important mediator involved in the host immune response, TLR4 is overexpressed in inflammation-associated colorectal neoplasia tissues, and contributes to the production of inflammatory factors in chronic colitis. Moreover, TLR4-deficient mice exhibit lower rates of colonic polyps (Fukata et al., 2007). Together, these results showed a critical role for the TLR4-mediated immune response in the tumorigenesis of colitis-associated CRC. Conversely, Lowe et al. reported that mice with colitis-associated colorectal cancer deficient in TLR2 easily developed significantly more and larger colorectal tumors compared with wild-type mice, together with increased expressions of IL-6, IL-17A, and phospho-STAT3 (Lowe et al., 2010). Inflammation was also widely reported in IBD-related CRC. Necrosis and regeneration of intestinal mucosa were the main pathological changes of IBD. Longer-duration chronic inflammation in IBD could lead to the abnormal regulation of the immune system (Kuraishy, Karin & Grivennikov, 2011). A number of proinflammatory pathways have been recently identified in inflammation-associated tumor development (Long, Lundsmith & Hamilton, 2017). Specific molecular signaling pathways such as NF-κB, has been implicated in the carcinogenesis of IBD-related CRC. NF-κB has been identified as a key upstream TF in regulating DEGs from ceRNA and NF-κB related pathway, a downstream part of LPS/TLR4, is highly enriched and popped up in KEGG pathway enrichment analysis. Deletion of IKKβ in intestinal epithelial cells decreased the incidence of colitis-associated cancer in the colitis-associated mouse cancer model (Greten et al., 2004). IL-6/STAT3 was also demonstrated to be involved in metabolic reprogramming, to promote the progression from chronic colitis to CRC (Qu et al., 2017), and the COX-2/PGE2 pathway was also shown to contribute to inflammation and carcinogenesis in IBD. Overexpression of COX-2 was observed in UC-associated neoplasia at both the gene and protein levels (Agoff et al., 2000). Peroxisome proliferator-activated receptor δ (PPARδ) was shown to exert an important role in chronic inflammation and pathophysiological processes of CRC via the COX-2/PGE2 pathway (Wang & DuBois, 2010; Wang et al., 2014). In vivo studies showed that PPAR δ was necessary for the production of proinflammatory mediators, such as chemokines, cytokines, COX-2, and PGE2, which led to colitis-associated tumor growth (Wang et al., 2014). In general, these immune response and inflammatory response-related hub genes identified in our study, including CXCL10, CSF1, CXCL1, CXCL9, IL1R1, IL1RN, CXCL11, FCGR1A, CXCL2 and GBP2 (centrality degree >10), deserve further research and discussion. Interestingly, all these genes are downregulated in CRC vs. IBD group, while they are all higher expressed in CRC and IBD compared to normal tissue. It seems that they are dynamically regulated according to the necessity of tumorigenesis process. Further research need be done to indicate the potential function of these genes in IBD related CRC.
We also identified three key ceRNAs, DPP10-AS1, DIO3OS and LINC01106, which were not ascertained in previous studies. Among them, LINC01106 is the only ceRNA related to overall survival of CRC patients. LIN01106 contained a transcript of 2204 bp with two exons located on chromosome 2q13. However, few studies have been conducted on the function of LINC01106. Our results showed intimate interactions between LINC01106 and miRNAs, such as MIR651, MIR193A, MIR140, MIR214, MIR34A, and MIR130A. Among them, MIR193A is a popular miRNA in scientific research and stands out for its divergent expression change in CRC vs. IBD relative to LINC01106 as well as its mRNA targets in the core ceRNA network. MIR193A, located on 17q11.2, has been widely found to be dysregulated in tumor progression. Lower expression of MIR193A was observed in human aldosterone-producing adrenocortical adenomas, and was functioned as a tumor suppressor by targeting and restraining CYP11B2 expression both at mRNA and protein level (Zhang et al., 2018). Another group demonstrated that MIR193A played an important role in the regulation of gastric cancer. Both gastric cell lines and human gastric tumors contained decreased levels of MIR193A. In addition, an in vitro study showed that upregulating MIR193A decreased tumor cell proliferation and migration, at least partly, by directly targeting cyclin D1 (CCND1) and ETS proto-oncogene 1 (ETS1) expression (Chou et al., 2018). Moreover, LINC00152, a lncRNA overexpressed in gastric cancer, was proved to sponge MIR193A to increase MCL1 expression thereby promoting cells proliferation (Huang et al., 2018). In ovarian cancer, MIR193A is silenced by methylated modification and down regulation of MIR193A expression promotes tumor aggressiveness by losing function of targeting GRB7 and MAPK/ERK pathways (Chen et al., 2018). Similar results were gained by another research that MIR193A is downregulated in metastatic prostate cancer and is involved in HOTAIR/EZH2/miR-193a feedback loop to exert a tumor suppressive function in prostate cancer (Ling et al., 2017). But, contradictorily, MIR193A increases the ability of chemoresistance in prostate cancer cell. MIR193A is noticed to be upregulated in prostate cancer tissues and cell lines, with significant suppression of cell apoptosis induced by oxidative stress by targeting Bach2 (Yang et al., 2017). The role of MIR193a in colorectal cancer and inflammatory bowel disease has also been broadly explored. In 2014, researchers demonstrated that MIR93A is downregulated in CRC to promote lymph node metastasis and result in poor survival (Zhang et al., 2014). In addition, MIR193A was evidenced to target and down regulate colonic PepT1, which reduced intestinal inflammation in response to microbiota (Dai et al., 2015). Another study confirms the tumor suppressor roles of MIR193A by perturbing KRAS function in colorectal adenocarcinoma, including reducing cell proliferation, increasing apoptosis, and inhibiting epithelial-mesenchymal transition (Mamoori et al., 2018). Moreover, MIR193A is revealed to be downregulated in ulcerative colitis neoplasia and promote tumorigenesis by releasing its control for IL17RD expression (Pekow et al., 2017). Considering the important roles of miRNAs in the development of CRC, we propose that our future research should therefore focus on the LINC01106-associated pathway and potential miRNA targets, especially MIR193A.
Our current study is based on published GEO datasets and TCGA COAD dataset. A limitation of the research is that we failed to enroll relevant mRNA, lncRNA, and microRNA by comparing IBD-induced CRC and IBD directly since it’s hard to find qualified data. We are trying to collect such kind of samples from clinical patients and will do the validation research in the future.
Conclusions
The present study identified a series of IBD-related mRNAs and lncRNAs that were potential mediators in the disease development from IBD to CRC. Using a series of bioinformatics analyses, lncRNA LINC01106, DPP10-AS1, and DIO3OS were identified, which were strongly associated with IBD-related CRC. By constructing a ceRNA network with lncRNAs, miRNAs, and mRNAs, we identified the links between these three RNA species. Further functional analyses suggested a potential mechanism through which these ceRNAs mediated tumorigenesis. Our results emphasized the important role of ceRNAs in the pathogenesis of IBD-related CRC, although more research is necessary to confirm our findings.
Supplemental Information
(A), (B) and (C): Distribution of gene expression levels in each group. (D), (E) and (F): Distribution of genes expression for each sample. QC, quality control.
(A–C): Volcano graph displaying pairs of expressed genes.
Acknowledgments
We are grateful to the researchers who provided their data for this analysis.
Abbreviation
- DEG
Differential expressed gene
- CRC
Colorectal cancer
- IBD
Inflammatory bowel disease
- HR
Hazard ratio
- UC
Ulcerative colitis
- CD
Crohn’s disease
- ceRNA
Competing endogenous RNA
- lncRNA
Long non-coding RNA
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- TF
Transcription factor
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Fangfang Sun conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper.
Weiwei Liang analyzed the data.
Kejun Tang contributed reagents/materials/analysis tools.
Mengying Hong contributed reagents/materials/analysis tools.
Jing Qian conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Data is available at The Cancer Genome Atlas via XENA (https://xenabrowser.net/datapages/?host=https%3A%2F%2Ftcga.xenahubs.net&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443) and GEO (GSE10714, GSE4183, and GSE68306).
References
- Agoff et al. (2000).Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC, Feng Z, Bronner MP. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. American Journal of Pathology. 2000;157:737–745. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad et al. (2017).Axelrad J, Kriplani A, Ozbek U, Harpaz N, Colombel JF, Itzkowitz S, Holcombe RF, Ang C. Chemotherapy tolerance and oncologic outcomes in patients with colorectal cancer with and without inflammatory bowel disease. Clinical Colorectal Cancer. 2017;16:e205–e210. doi: 10.1016/j.clcc.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart & Sandborn (2012).Baumgart DC, Sandborn WJ. Crohn’s disease. The Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- Bopanna et al. (2017).Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2017;2:269–276. doi: 10.1016/S2468-1253(17)30004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen K, Liu MX, Mak CS, Yung MM, Leung TH, Xu D, Ngu SF, Chan KK, Yang H, Ngan HY, Chan DW. Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways. Theranostics. 2018;8:423–436. doi: 10.7150/thno.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou et al. (2018).Chou NH, Lo YH, Wang KC, Kang CH, Tsai CY, Tsai KW. MiR-193a-5p and -3p play a distinct role in gastric cancer: mir-193a-3p suppresses gastric cancer cell growth by targeting ETS1 and CCND1. Anticancer Research. 2018;38:3309–3318. doi: 10.21873/anticanres.12596. [DOI] [PubMed] [Google Scholar]
- Chowdhury, Sacks & Sheerin (2006).Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clinical and Experimental Immunology. 2006;145:346–356. doi: 10.1111/j.1365-2249.2006.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai et al. (2015).Dai X, Chen X, Chen Q, Shi L, Liang H, Zhou Z, Liu Q, Pang W, Hou D, Wang C, Zen K, Yuan Y, Zhang CY, Xia L. MicroRNA-193a-3p reduces intestinal inflammation in response to microbiota via down-regulation of colonic PepT1. Journal of Biological Chemistry. 2015;290:16099–16115. doi: 10.1074/jbc.M115.659318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese & Fiocchi (2011).Danese S, Fiocchi C. Ulcerative colitis. New England Journal of Medicine. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- Eaden, Abrams & Mayberry (2001).Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata et al. (2007).Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten et al. (2004).Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Huang, Sherman & Lempicki (2008a).Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research. 2008a;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Sherman & Lempicki (2008b).Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2008b;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang Y, Luo H, Li F, Yang Y, Ou G, Ye X, Li N. LINC00152 down-regulated miR-193a-3p to enhance MCL1 expression and promote gastric cancer cells proliferation. Bioscience Reports. 2018;38 doi: 10.1042/BSR20171607. Article BSR20171607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Josse & Bours (2016).Josse C, Bours V. MicroRNAs and inflammation in colorectal cancer. Advances in Experimental Medicine and Biology. 2016;937:53–69. doi: 10.1007/978-3-319-42059-2_3. [DOI] [PubMed] [Google Scholar]
- Kameyama et al. (2018).Kameyama H, Nagahashi M, Shimada Y, Tajima Y, Ichikawa H, Nakano M, Sakata J, Kobayashi T, Narayanan S, Takabe K, Wakai T. Genomic characterization of colitis-associated colorectal cancer. World Journal of Surgical Oncology. 2018;16 doi: 10.1186/s12957-018-1428-0. Article 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan et al. (2012).Kanaan Z, Rai SN, Eichenberger MR, Barnes C, Dworkin AM, Weller C, Cohen E, Roberts H, Keskey B, Petras RE, Crawford NP, Galandiuk S. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Human Mutation. 2012;33:551–560. doi: 10.1002/humu.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman et al. (2007).Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical Gastroenterology and Hepatology. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Khor, Gardet & Xavier (2011).Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishy, Karin & Grivennikov (2011).Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis et al. (2017).Lewis A, Felice C, Kumagai T, Lai C, Singh K, Jeffery RR, Feakins R, Giannoulatou E, Armuzzi A, Jawad N, Lindsay JO, Silver A. The miR-200 family is increased in dysplastic lesions in ulcerative colitis patients. PLOS ONE. 2017;12(3):e0173664. doi: 10.1371/journal.pone.0173664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Research. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling et al. (2017).Ling Z, Wang X, Tao T, Zhang L, Guan H, You Z, Lu K, Zhang G, Chen S, Wu J, Qian J, Liu H, Xu B, Chen M. Involvement of aberrantly activated HOTAIR/EZH2/miR-193a feedback loop in progression of prostate cancer. Journal of Experimental & Clinical Cancer Research. 2017;36 doi: 10.1186/s13046-017-0629-7. Article 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Lundsmith & Hamilton (2017).Long AG, Lundsmith ET, Hamilton KE. Inflammation and colorectal cancer. Current Colorectal Cancer Reports. 2017;13:341–351. doi: 10.1007/s11888-017-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe et al. (2010).Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, Shimada K, Wong MH, Michelsen KS, Arditi M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLOS ONE. 2010;5:e13027. doi: 10.1371/journal.pone.0013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoori et al. (2018).Mamoori A, Wahab R, Islam F, Lee K, Vider J, Lu CT, Gopalan V, Lam AK. Clinical and biological significance of miR-193a-3p targeted KRAS in colorectal cancer pathogenesis. Human Pathology. 2018;71:145–156. doi: 10.1016/j.humpath.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Ng et al. (2018).Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- Ng et al. (2013).Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, De Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL. Asia-Pacific Cs and Colitis Epidemiologic Study Study G Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Pekow et al. (2017).Pekow J, Meckel K, Dougherty U, Huang Y, Chen X, Almoghrabi A, Mustafi R, Ayaloglu-Butun F, Deng Z, Haider HI, Hart J, Rubin DT, Kwon JH, Bissonnette M. miR-193a-3p is a key tumor suppressor in ulcerative colitis-associated colon cancer and promotes carcinogenesis through upregulation of IL17RD. Clinical Cancer Research. 2017;23:5281–5291. doi: 10.1158/1078-0432.CCR-17-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu et al. (2017).Qu D, Shen L, Liu S, Li H, Ma Y, Zhang R, Wu K, Yao L, Li J, Zhang J. Chronic inflammation confers to the metabolic reprogramming associated with tumorigenesis of colorectal cancer. Cancer Biology & Therapy. 2017;18:237–244. doi: 10.1080/15384047.2017.1294292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie et al. (2015).Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena et al. (2011).Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardoni et al. (0000).Scardoni G, Laudanna C, Tosadori G, Fabbri F, Faizaan M. CentiScaPe: network centralities for cytoscape. 0000. [DOI] [PMC free article] [PubMed]
- Shawki et al. (2018).Shawki S, Ashburn J, Signs SA, Huang E. Colon Cancer: inflammation-associated cancer. Surgical Oncology Clinics of North America. 2018;27:269–287. doi: 10.1016/j.soc.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svrcek et al. (2013).Svrcek M, El-Murr N, Wanherdrick K, Dumont S, Beaugerie L, Cosnes J, Colombel JF, Tiret E, Flejou JF, Lesuffleur T, Duval A. Overexpression of microRNAs-155 and 21 targeting mismatch repair proteins in inflammatory bowel diseases. Carcinogenesis. 2013;34:828–834. doi: 10.1093/carcin/bgs408. [DOI] [PubMed] [Google Scholar]
- Szklarczyk et al. (2018).Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2018;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2017).Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Research. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thia et al. (2008).Thia KT, Loftus Jr EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. American Journal of Gastroenterology. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- Vegh, Kurti & Lakatos (2017).Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. Journal of Digestive Diseases. 2017;18:92–98. doi: 10.1111/1751-2980.12449. [DOI] [PubMed] [Google Scholar]
- Wang & DuBois (2010).Wang D, DuBois RN. Therapeutic potential of peroxisome proliferator-activated receptors in chronic inflammation and colorectal cancer. Gastroenterology Clinics of North America. 2010;39:697–707. doi: 10.1016/j.gtc.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang D, Fu L, Ning W, Guo L, Sun X, Dey SK, Chaturvedi R, Wilson KT, DuBois RN. Peroxisome proliferator-activated receptor delta promotes colonic inflammation and tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7084–7089. doi: 10.1073/pnas.1324233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee et al. (2012).Wee LM, Flores-Jasso CF, Salomon WE, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151:1055–1067. doi: 10.1016/j.cell.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang Z, Chen JS, Wen JK, Gao HT, Zheng B, Qu CB, Liu KL, Zhang ML, Gu JF, Li JD, Zhang YP, Li W, Wang XL, Zhang Y. Silencing of miR-193a-5p increases the chemosensitivity of prostate cancer cells to docetaxel. Journal of Experimental & Clinical Cancer Research. 2017;36 doi: 10.1186/s13046-017-0649-3. Article 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2011).Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Research. 2011;39:D202–D209. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi et al. (2016).Yi Y, Zhao Y, Li C, Zhang L, Huang H, Li Y, Liu L, Hou P, Cui T, Tan P. RAID v2.0: an updated resource of RNA-associated interactions across organisms. Nucleic Acids Research. 2016;45:D115–D118. doi: 10.1093/nar/gkw1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang P, Ji DB, Han HB, Shi YF, Du CZ, Gu J. Downregulation of miR-193a-5p correlates with lymph node metastasis and poor prognosis in colorectal cancer. World Journal of Gastroenterology. 2014;20:12241–12248. doi: 10.3748/wjg.v20.i34.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang G, Zou X, Liu Q, Xie T, Huang R, Kang H, Lai C, Zhu J. MiR-193a-3p functions as a tumour suppressor in human aldosterone-producing adrenocortical adenoma by down-regulating CYP11B2. International Journal of Experimental Pathology. 2018;99:77–86. doi: 10.1111/iep.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2016).Zhu M, Li D, Jin M, Li M. Association between microRNA polymorphisms and the risk of inflammatory bowel disease. Molecular Medicine Reports. 2016;13:5297–5308. doi: 10.3892/mmr.2016.5157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A), (B) and (C): Distribution of gene expression levels in each group. (D), (E) and (F): Distribution of genes expression for each sample. QC, quality control.
(A–C): Volcano graph displaying pairs of expressed genes.
Data Availability Statement
The following information was supplied regarding data availability:
Data is available at The Cancer Genome Atlas via XENA (https://xenabrowser.net/datapages/?host=https%3A%2F%2Ftcga.xenahubs.net&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443) and GEO (GSE10714, GSE4183, and GSE68306).