Fig. 1.
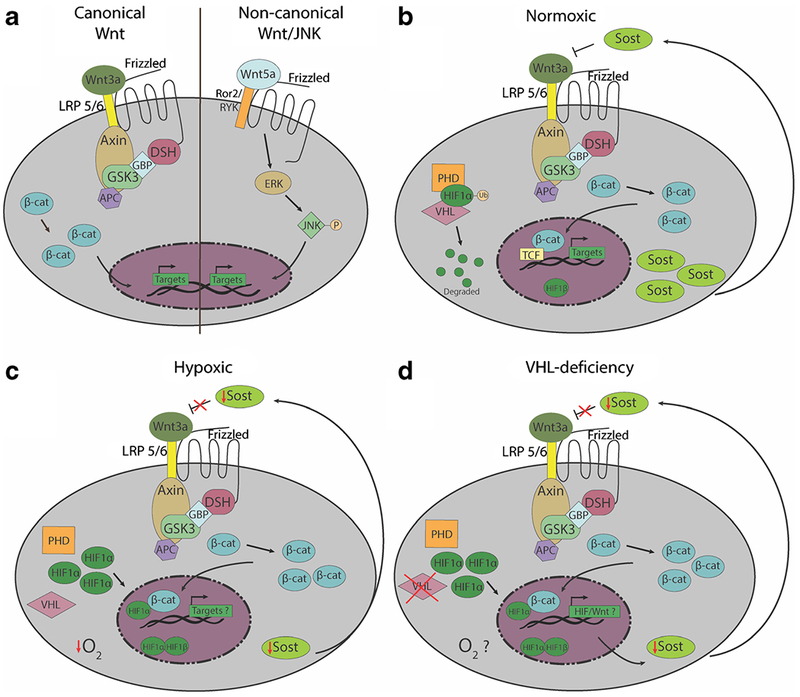
Overview of Wnt signaling pathways and the regulation by Wnt antagonists and the bone microenvironment. a Schematic of canonical (left) and non-canonical (right) Wnt pathways. In canonical Wnt signaling, binding of ligands like Wnt3a to Frizzled family receptors results in the release of β-catenin from the Axin/GSK3/APC/DSH/GBP complex, allowing β-catenin to translocate from the cytoplasm into the nucleus for Wnt target gene transcription. In the non-canonical Wnt/JNK pathway, β-catenin is not utilized. Non-canonical Wnt ligands such as Wnt5a also bind to Frizzled receptors, but utilize the Ror2/Ryk coreceptors to activate downstream phosphorylation of JNK by ERK. Phosphorylated JNK then translocates into the nucleus to activate Wnt target gene transcription. b Under normoxic conditions, PHDs hydroxylate HIF1α, allowing von Hippel-Lindau (Vhl) protein to ubiquitinate Hif1a, which is subsequently degraded. In normal conditions SOST binds to LRP 5/6 and antagonizes Wnt3a c Under hypoxic conditions, PHDs do not hydroxylate HIF1α, and VHL protein is inactivated. Therefore, HIF1α is not ubiquitinated or degraded, and is translocated into the nucleus to bind HIF1β to activate transcription of hypoxia-induced genes. Genetos et al. demonstrated that under hypoxic conditions, SOST is decreased in osteoblasts, which could lead to an increase in canonical Wnt signaling. There is evidence that Hif1α and β-catenin can act as co-activators, but how hypoxia affects Wnt signaling is not well understood. d In Dmp-Cre driven Vhl-knockout mice, which have been used as a model to study the effects of Hif1α stabilization in osteocytes in vivo, Sost expression is diminished. In turn, this decrease may may result in increased canonical Wnt signaling and promote the building of bone mass. However, whether the osteocytes or the other cells within the bone and marrow microenvironments of Vhl-conditional knockout are truly hypoxic awaits further investigation.
