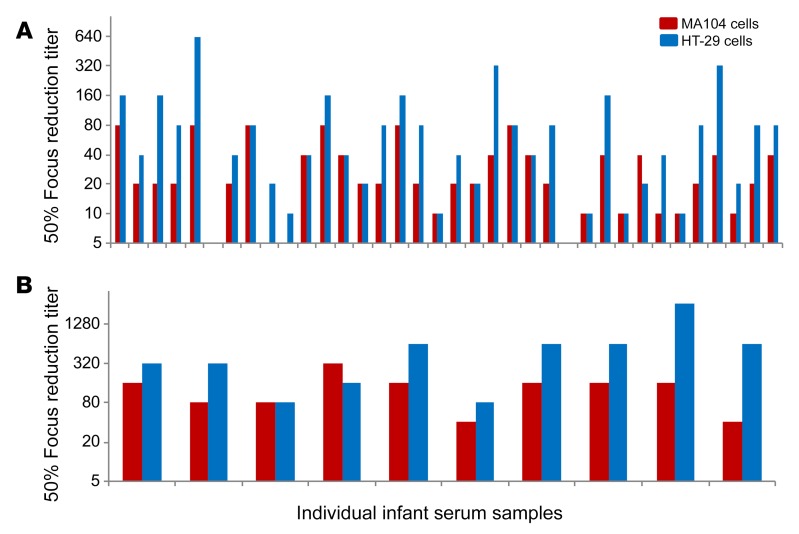
Figure 5. Neutralization titers of post–RV1 vaccination serum samples from Indian or US infants measured in MA104 or HT-29 cells.
Diluted infant serum samples (in duplicate) and Wa (G1P[8]) mixtures were added to MA104 or HT-29 cells and incubated at 37°C. At 16 hours after infection, RV FFUs were determined by immunostaining using a rabbit polyclonal anti-RV antibody. Focus reduction titers were defined as the maximum serum dilution that resulted in a 50% or greater focus reduction. (A) Infant serum samples from India RV1 vaccine study (45). (B) Infant serum samples from US RV vaccine study (46, 47). Infant serum samples from the US were tested 2 times with similar results and infant serum samples from India were tested only once due to small sample volumes. The neutralization titer differences between MA104 and HT-29 cells were statistically significant (P = 0.01 by t test of means of log2-transformed titers for both Indian and US samples).