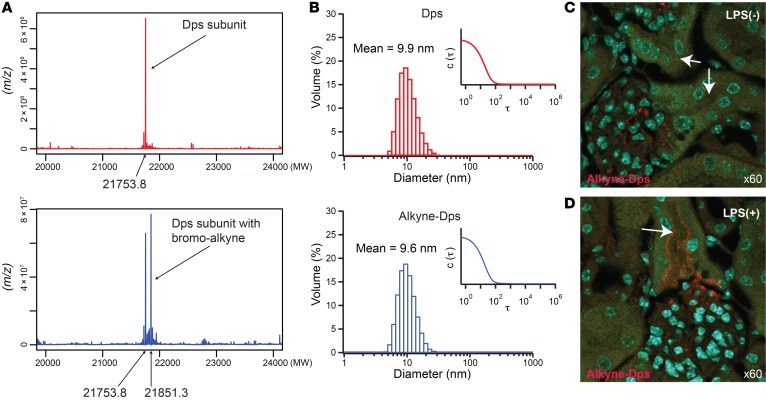
Figure 7. Modulation of surface characteristics and its effects on biodistribution.
(A) Synthesis of alkyne-Dps. Deconvoluted mass spectrometry data for Dps subunit (MW, 21753.8) and bromo-alkyne–labeled Dps subunit (N-propargyl bromoacetamide; MW, 21851.3) are shown. (B) Volume-averaged hydrodynamic diameter and corresponding correlation function (inset) of Dps and that labeled with N-propargyl bromoacetamide (alkyne-Dps) measured with dynamic light scattering. (C and D) Differential distribution of alkyne-Dps in vivo with and without LPS challenge is shown (red). Arrows point to S1 proximal tubules where alkyne-Dps was detected only in LPS-challenged mice. Alkyne-Dps and LPS were administered i.v. and i.p., respectively, 4 hours before tissue harvest. To visualize alkyne-Dps, Alexa Fluor 555–azide was conjugated to alkyne-Dps using copper(I)-catalyzed azide-alkyne cycloaddition (click chemistry) after tissue harvest. Nuclei were stained blue with DAPI; green shows autofluorescence.