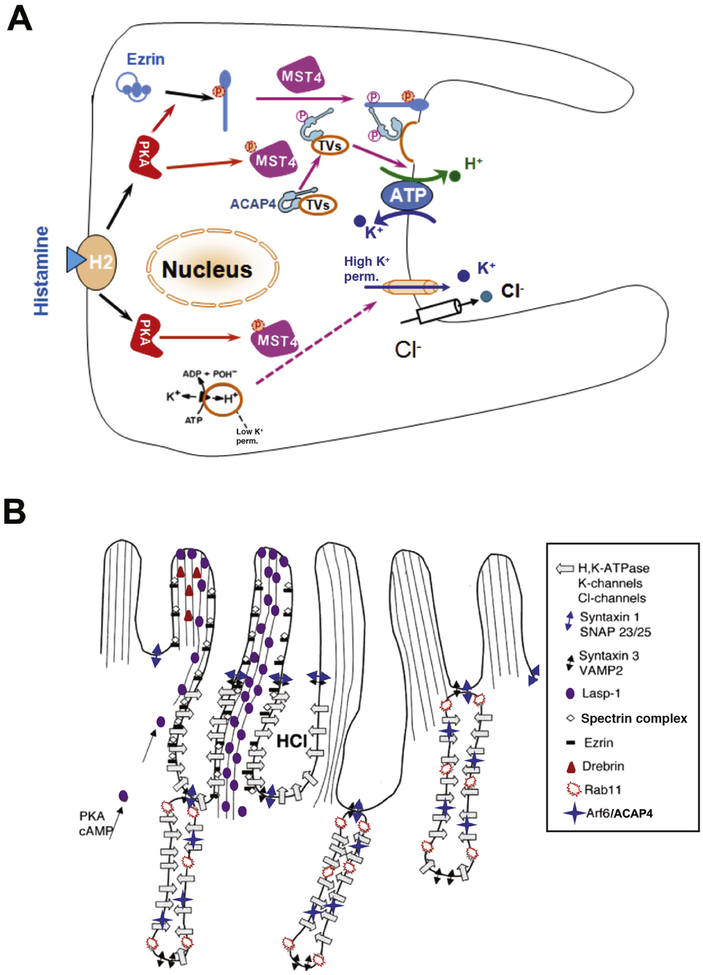
Figure 1.
Mechanisms of parietal cell acid secretion. (A) In the resting state, H,K-ATPase and ACAP4 are sequestered in tubulovesicles with low K+ permeability. Histamine stimulation activates PKA signaling via MST4, resulting in conformational changes in ezrin and ACAP4 that allow them to interact and induce tubulovesicle translocation. MST4 then phosphorylates ACAP4 at Thr545, promoting its association with ezrin. Phosphorylation of ezrin by PKA at Ser66 causes a conformational change, which promotes docking and anchoring of H,K-ATPase- and ACAP4-containing tubulovesicles at the apical plasma membrane, where K+ permeability is high. MST4 might mediate K+ channel activation, but the mechanisms of this process have not been determined. (B) Model of apical membrane cytoskeletal remodeling. Histamine stimulation activates PKA, resulting in phosphorylation and activation of MST4 kinase. Activated MST4 mediates formation of the apical signaling machinery, comprising ARF6, ACAP4, and ezrin, which docks tubulovesicles to the apical membrane by interactions with Lasp-1, drebrin, spectrin, and other proteins, such as syntaxin 1 and SNAP23/25. This leads to reorganization of apical microvillous structures with extended apical surface and onset of proton pumping. The exact mechanisms underlying tubulovesicle fusion with the apical membrane and insertion of proton pump into the apical membrane remain less illustrated.