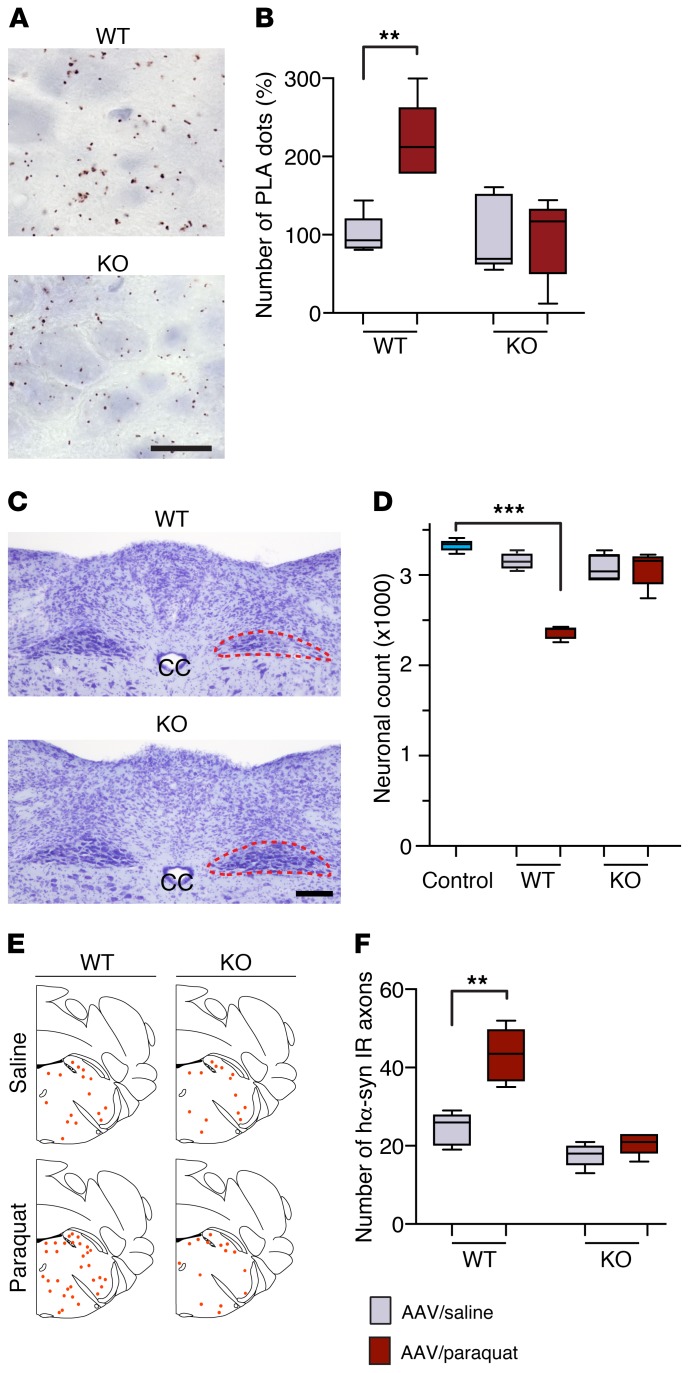
Figure 7. Gp91phox-deficient mice are resistant to paraquat-induced DMnX pathology.
WT and gp91phox-deficient (KO) mice received an injection of hα-synuclein AAVs into the left vagus nerve and were injected systemically with either saline (n ≥ 4/genotype, gray bars) or paraquat (n ≥ 4/genotype, red bars) and sacrificed at 7 days. (A) Medullary tissue sections were processed for indirect hα-synuclein/3-NT PLA. Representative images show the left DMnX of a WT and a KO mouse injected with hα-synuclein AAVs/paraquat. Specific PLA signal was apparently reduced in the KO mouse. Scale bar: 10 μm. (B) The number of hα-synuclein/3-NT PLA dots was counted in the left DMnX of WT and KO mice injected with hα-synuclein AAVs/saline or hα-synuclein AAVs/paraquat. Values are expressed as percentage of the mean value in the corresponding hα-synuclein AAV/saline–injected animals. (C) Representative images of Nissl-stained neurons in the dorsal medulla oblongata. Tissues were obtained from a WT and a KO mouse injected with hα-synuclein AAVs/paraquat. The left DMnX is delineated in red. Neuronal loss is evident only in the left (ipsilateral to AAV infusion) DMnX from the WT animal. Scale bar: 100 μm. (D) The number of Nissl-stained neurons was counted stereologically in the left DMnX of WT and KO mice treated with hα-synuclein AAVs/saline or hα-synuclein AAVs/paraquat. Control counts (n = 5, light blue bar) were obtained from the right DMnX of WT animals injected with hα-synuclein AAVs/saline. (E and F) Pontine tissue sections were immunostained with anti–hα-synuclein. The schematic plots show the distribution of hα-synuclein–labeled axons in the left pons. In the graph, data show the counts of hα-synuclein–positive axons in the left pons. Box and whisker plots show median, upper and lower quartiles, and maximum and minimum as whiskers. **P ≤ 0.01; ***P ≤ 0.001, Mann-Whitney U test (saline- versus paraquat-injected mice) or Kruskal-Wallis followed by Dunn’s post hoc (D).