Figure 1.
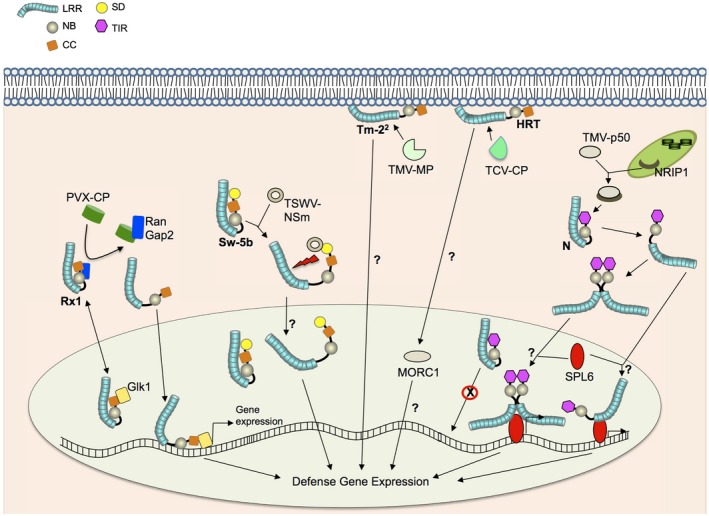
Recognition of viral pathogens by intracellular NLRs. In the absence of infection, the Rx1 CC‐NLR exists in an autoinhibited state stabilized by RanGAP2. Upon recognition of the PVX coat protein (PVX‐CP), Rx1 releases its autoinhibition and enters the nucleus, where it associates with the Golden2‐like transcription factor NbGlk1 to promote the transcription of defence genes. The SD and NB‐LRR domains of the Sw‐5b CC‐NLR interact with the movement protein NSm of TSWV to relieve auto‐inhibition and activate the defence response. Sw‐5b is present in the nucleus in the absence of infection but its role in the nucleus is unknown. It is not known if activated Sw‐5b moves into the nucleus. TM‐22 and HRT are membrane‐associated CC‐NLRs; TM‐22 recognizes the movement protein of tobamoviruses including TMV (TMV‐MP), and HRT recognizes CP of TCV (TCV‐CP). HRT and Rx1 (not shown) interact with MORC1, which has an unknown nuclear function. The nucleocytoplasmic TIR‐NLR N confers resistance to TMV through recognition of the 50 kDa helicase domain of the TMV replicase (TMV‐p50). Recognition of p50 by N requires NRIP1, which relocalizes from the chloroplast to the cytoplasm during infection. Following p50 recognition, N undergoes self‐association; it also associates with the transcription factor SPL6 in the nucleus to promote transcription of defence genes. It is currently unknown which activated form (monomeric or self‐associated N) associates with SPL6 in the nucleus.
