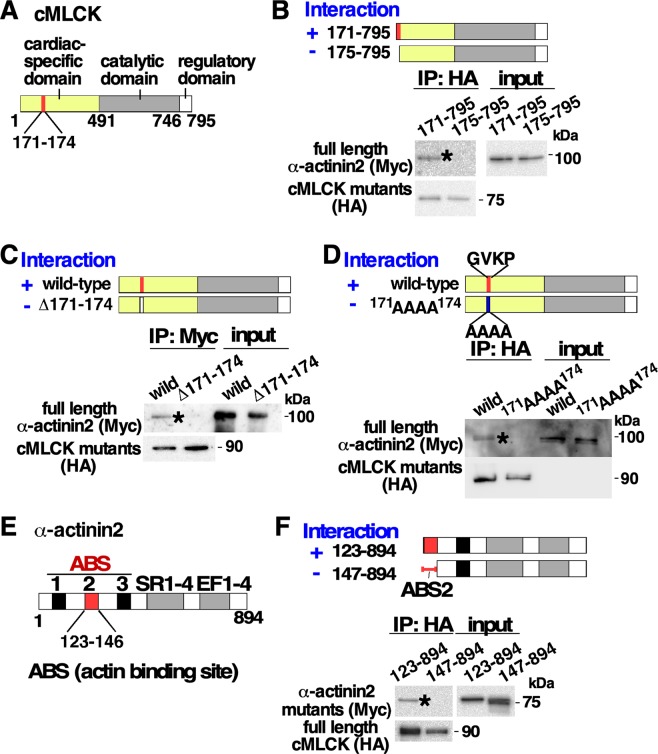
Figure 3.
Identification of four amino acids located in the domain unique to the cardiac isoform required for interacting with α-actinin2. (A) Schematics of cMLCK protein including four amino acids 171–174 at the N-terminus region specific for cardiac isoform. (B) Two N-terminus deletion mutants, HA-tagged cMLCK(171–795) and cMLCK(175–795), were mixed with Myc-tagged full-length α-actinin2. Presence ( + ) and absence (−) of interaction between two proteins were indicated. (C) Internal deletion mutant of cMLCK (Δ171–174), and (D) four amino acid substituted mutants from 171DVKP174 to 171AAAA174 were mixed with Myc-tagged full-length α-actinin2. (E) Schematics of α-actinin2 protein including the second actin binding site. (F) Two Myc-tagged N-terminus deletion mutants of α-actinin2 with or without ABS2 domain were mixed with HA-full-length cMLCK. The additional results were available in Supplemental Fig. 1. ABS, F-actin binding site; SR, spectrin repeat; EF, EF hand motif.