Figure 5.
BRAF Inhibition Enhances the Effect of DNA Damage-Based Therapy in CRC Patient-Derived Tumoroids
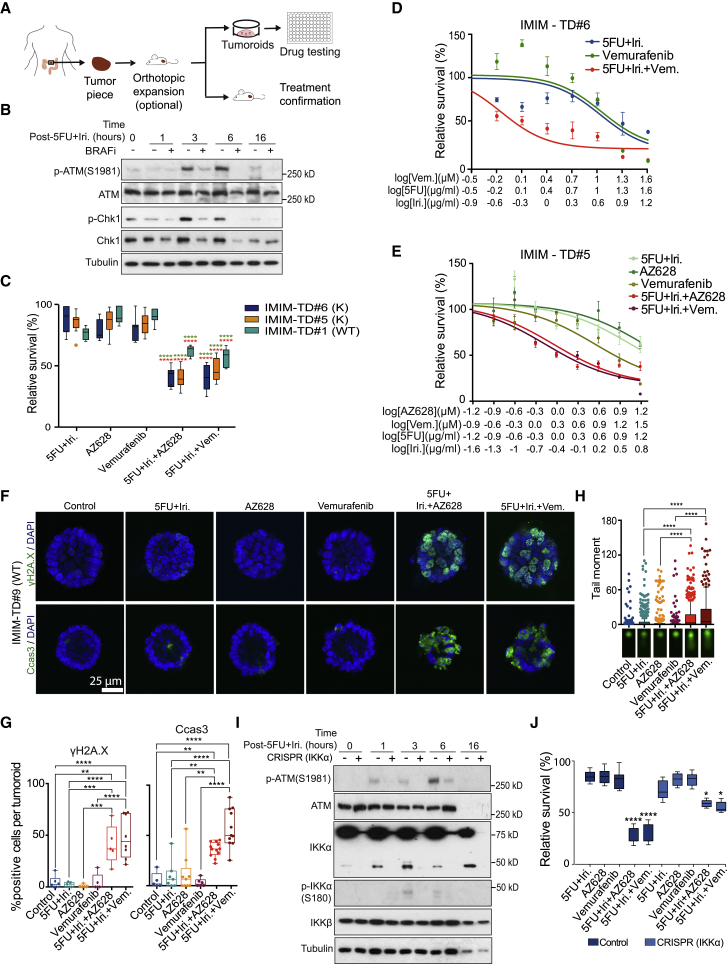
(A) Experimental design used for the expansion of primary CRC tumors, generation of patient-derived tumoroids, and drug testing.
(B) Western blot analysis of control and BRAF-inhibited IMIM-TD#5, collected at the indicated time points after IR treatment.
(C) Quantification of tumoroid viability after treatment with the indicated compounds as a single treatment or in combination.
(D and E) Dose-response curves of two different tumoroids, IMIM-TD#6 (D) and IMIM-TD#5 (E), treated as indicated.
(F and G) Representative images of γH2A.X and cleaved caspase 3 staining in a representative tumoroid treated as indicated (F) and quantification of the percentage of positive cells from 20 tumoroids per condition counted (G).
(H) Comet assay of IMIM-TD#9, treated as indicated.
(I and J) Western blot analysis of control and IKKα-depleted IMIM-TD#9 using CRISPR-Cas9 technology (I) and quantification of cell viability after 72 h of culture with the indicated treatments (J).
The statistical analysis in (C) and (I) was performed by unpaired t test, comparing the combination treatments with single 5FU+irinotecan (red) or BRAF/IKK inhibitors (green); ∗∗∗∗p < 0.0001, n.s., non-significant. For the statistical analysis in (G), we used two-way ANOVA, and the p values are indicated as ∗∗p < 0.01 and ∗∗∗∗p < 0.0001. Vem, vemurafenib; 5FU, 5-fluorouracil; Dox, doxorubicin; and Iri, irinotecan.
See also Figure S5.