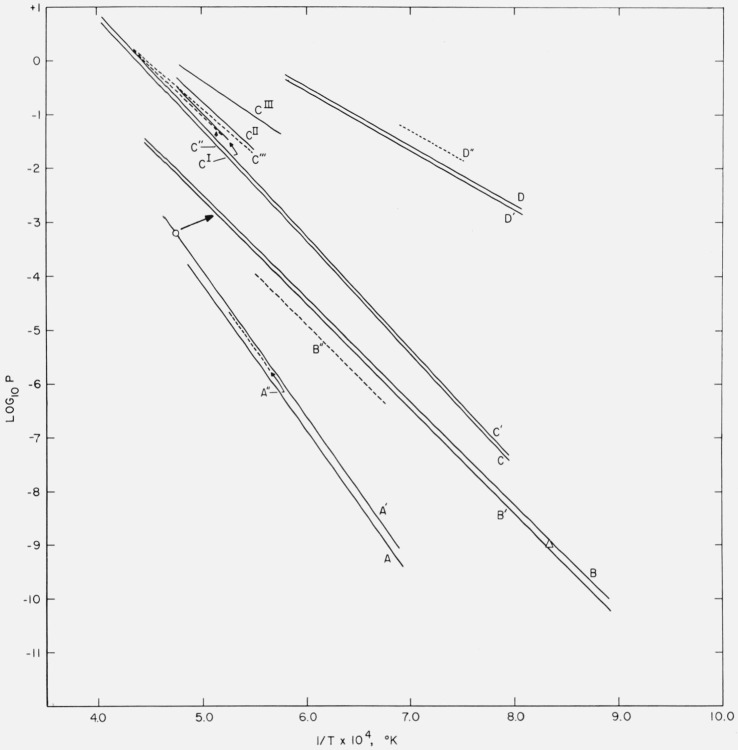
Figure 5. Comparison of vapor pressures (log10 P versus 1/T) calculated from heat of formation and thermodynamic data with published values obtained in high-temperature equilibrium measurements.
(a) 4AlN(c) + 3C(c) → Al4C3(c) + 2N2(g): A and A′ calculation from thermal data,* A′′ = Prescott and Hincke [36].
(b) Al4C3(c) → 4Al(g) + 3C(c): B and B′ = calculation from thermal data,* B′′ = Meschi and Searcy [31], ○ = Chupka et al. [34], Δ = Campbell [35].
(c) 2Al2O3(c) + 9C(c) = Al4C3(c) + 6CO(g): C and C′ calculation from thermal data,*
C′′ = Prescott and Hincke [37], C′′′ = Brunner [38].
CI = Al4O4C(c) + 6C(c) → Al4C3(c) + 4CO(g): Cox and Pidgeon [40].
CII = 2Al2O3(c) + 3C(c) → Al4O4C(c) + 2CO(g): [40].
CIII = Al2O3(c) + 3C(c) → Al2OC(c) + 2CO(g): [40].
(d) 8MgO(c) + Al4C3(c) → 2MgAl2O4(c) + 3C(c) + 6Mg(g): D and D′ = calculation from thermal data, D′′ = Grjotheim et al. [41].