Abstract
The treatment of several solid and hematologic malignancies with immune checkpoint inhibitors (against programmed death receptor-1/ligand-1 [PD-1/PD-L1]) has dramatically changed the cancer treatment paradigm. However, no checkpoint inhibitors were previously approved for the treatment of triple-negative breast cancer (TNBC), a difficult-to-treat disease with a high unmet therapeutic need. Based on IMpassion130 clinical trial (NCT02425891), the Food and Drug Administration (FDA) has recently granted an accelerated approval for atezolizumab (TECENTRIQÒ), a monoclonal antibody drug targeting PD-L1, plus chemotherapy (Abraxane; nabÒ-Paclitaxel) for the treatment of adults with PD-L1-positive, unresectable, locally advanced or metastatic TNBC. The FDA has also approved the Ventana diagnostic antibody SP142 as a companion test for selecting TNBC patients for treatment with atezolizumab. In the present review, we briefly discuss the importance of this breakthrough as the first cancer immunotherapy regimen to be approved for the management of breast cancer.
Keywords: Breast cancer, triple-negative breast cancer, immune checkpoint inhibitors, predictive biomarkers, PD-L1
REVIEW
Recent advances in the development of cancer immunotherapy using immune checkpoint inhibitors against either programmed death receptor-1 (PD-1) or its ligand PD-L1 have revolutionized treatment of several solid tumors [1-4]. The interaction between PD-1 on T-cells and its ligands PD-L1 and PD-L2 on cancer cells promotes T-cell exhaustion and conversion of T effector cells to immunosuppressive T regulatory (Treg) cells (Figure 1) [5]. Immune checkpoint inhibitors (against either PD-1 or PD-L1) block the suppressor PD-1/PD-L1 axis contributing to the reactivation of cytotoxic T effector cells and consequently enhancing the anticancer activity of the immune system (Figure 1) [5].
FIGURE 1.
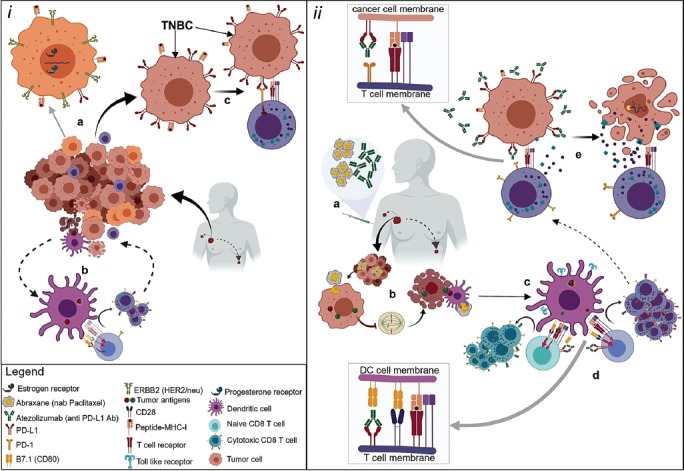
The possible mechanism of action of anti-programmed death receptor-1/ligand-1 (PD-1/PD-L1) drugs (e.g., atezolizumab) and nab-paclitaxel in metastatic triple-negative breast cancer (TNBC). (i) Metastatic breast cancers are dynamic environments undergoing proliferation and apoptosis along with immune cell infiltration. Breast cancers are heterogeneous populations which may or may not (e.g., TNBC) be influenced by ERBB2/human epidermal growth factor receptor 2 (HER2) and/or nuclear estrogen receptor (ER) and progesterone receptor (PR) signaling (a). Apoptotic tumor cells are phagocytosed, and tumor-specific antigens are expressed on the major histocompatibility complex (MHC) molecules by tumor-infiltrating antigen presenting cells (APC) that may activate antigen-specific cytotoxic T cell (CTL) responses by engaging their T cell receptors. Costimulatory signals through B7 on APCs and CD28 on T cells are necessary for CTL differentiation and proliferation. T cells express PD-L1 and its receptor PD-1. The interaction of PD-L1 with B7.1 suppresses T cell differentiation into CTL (b). Metastatic TNBC expresses PD-L1 in abundance. The engagement of PD-L1 with PD-1 blocks CTL effector function allowing unchecked tumor progression (c). (ii) Metastatic TNBC positive for PD-L1 respond to combination therapy with atezolizumab (monoclonal anti-PD-L1 antibody) and Abraxane (nab-paclitaxel) (a). Abraxane enhances expression of tumor-specific antigens and induction of apoptosis (G2/M phase arrest), thereby facilitating antigen presentation via APCs (b). APCs are activated via nab-paclitaxel and enhance toll-like receptor (TLR) expression contributing to efficient antigen presentation as well as costimulation by B7 family (c). Suppressive signal in activating T cells is inhibited as atezolizumab blocks the interaction of PD-L1 with B7.1. Thus augmenting signal induction for activation, differentiation, and proliferation of tumor-specific CTL clones (d). Metastatic TNBC expresses PD-L1 in abundance. Disrupting the engagement of PD-L1 with its receptor PD-1 by anti-PD-L1 antibody allows CTLs to secrete perforins that make holes in the target cell membrane allowing granzymes to enter and induce caspase-dependent apoptosis of these aggressive cancers (e).
Stunning successes of monoclonal antibody-based immune checkpoint inhibitors against PD-1/PD-L1 (e.g., nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, and ipilimumab) have been achieved in various cancers [6]. These include non-small cell lung carcinoma (NSCLC), renal, bladder, head and neck, gastric/gastroesophageal junction (GEJ), microsatellite instable (MSI-H) colorectal, cervical, hepatocellular and Merkel cell carcinoma, as well as in malignant melanoma (both pediatric and adult) and classical Hodgkin’s lymphoma [6,7]. In addition, an anti-PD-1 agent pembrolizumab has been approved for all solid MSI-H cancers regardless the histotype (“tumor agnostic approach”) [7-10].
Triple-negative breast cancer (TNBC) is a complex and highly aggressive subtype of breast cancer lacking estrogen (ER), progesterone (PR), and human epidermal growth factor receptor 2 (HER2) receptors, thereby making it difficult to treat [11]. It carries the highest metastatic potential and has the poorest clinical outcome among all the subtypes of breast cancer [11]. Due to the advances in molecular characterization of TNBC, various novel therapeutic targets including poly ADP-ribose polymerase-1 (PARP-1) inhibitors, tyrosine kinase inhibitors, immune checkpoints, anti-androgens, and epigenetic targets have come into focus [11].
Although breast cancer has been initially considered a “non-immunogenic” cancer, numerous studies have now shown PD-L1 expression in both cancer and inflammatory cells (tumor infiltrating lymphocytes [TILs]). PD-L1 positivity in cancer or inflammatory cells has been reported across the breast cancer histotypes [12-26]. In particular, ER-negative breast cancers (TNBC and HER2 positive) have been shown to be “immunogenic” and potentially amenable for the trials with anti-PD-1/PD-L1 agents [12,16,17,20,26-35]. TNBC is thought to have relatively high PD-L1 expression, predominantly in inflammatory (immune) cells and occasionally in cancer cells (Figure 2A and B) [14,16-18,20,29-31,36-39]. A recent systematic review of Zhang et al. [40], based on analysis >2500 breast cancers, revealed PD-L1 positivity in the range 21-56% (Table 1). In such studies, the tumor proportion score (TPS) is defined as “the percentage of viable tumor cells showing partial or complete membrane staining (≥1+) relative to all viable tumor cells present in the sample (positive and negative)”, source: https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150013b.pdf, accessed: March 10, 2019). It is noteworthy that, in most PD-L1-positive breast cancers, PD-L1 expression is not diffuse (>50% of positive cancer cells or TPS <50%) but rather focal or patchy and limited to a small proportion of cancer cells [14].
FIGURE 2.
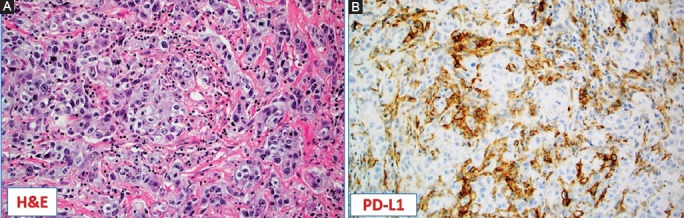
Programmed death ligand-1 (PD-L1) as a biomarker for triple-negative breast cancer (TNBC). (A) Hematoxylin and Eosin (H&E) slide of the high-grade, TNBC not otherwise specified (NOS) with abundant tumor infiltrating lymphocytes (TILs) [×10]; (B) TILs were strongly positive for PD-L1 by immunohistochemistry (clone SP142, Ventana). Note the lack of PD-L1 expression in the cancer cells (×10).
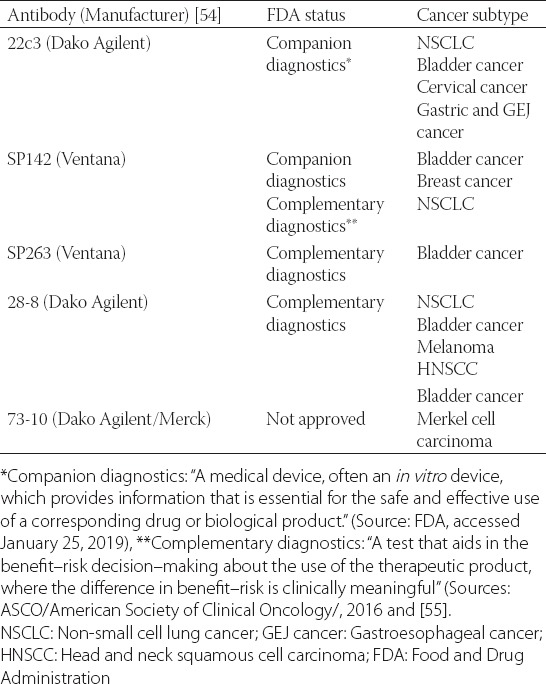
TABLE 1.
A list of the available diagnostic anti-PD-L1 antibodies and their status in regards to the FDA approval
There are numerous ongoing clinical trials with immune checkpoint inhibitors combined with other treatment modalities in breast cancer [41]. Of note has been atezolizumab which selectively targets PD-L1 to prevent interaction with the receptors PD-1 and B7-1 (a costimulatory cell-surface protein) to reverse T-cell suppression. It had previously been approved as a single-agent for the treatment of metastatic urothelial/bladder carcinoma and non–small-cell lung cancer [42,43]. This monoclonal antibody drug is clinically effective and has a good safety profile in patients with other solid tumors [1], so it was a good candidate for potential use in the management of patients with TNBC. The most remarkable results have been recently achieved in the clinical trial IMpassion130 (NCT02425891) [44]. This study led the Food and Drug Administration (FDA) to grant accelerated approval for atezolizumab (TECENTRIQÒ, anti-PD-L1 agent) in combination with chemotherapy using a solvent-free, nanoparticle albumin-bound (nab) formulation of paclitaxel (nabÒ-Paclitaxel; Abraxane) for the treatment of patients with TNBC (date of approval: March 8, 2019). The approval was based on the randomized trial involving 902 TNBC patients with unresectable, locally advanced, and/or metastatic TNBC without prior treatment for the metastatic disease. Patients were randomized (1:1) to receive atezolizumab plus chemotherapy with Abraxanevs. placebo plus Abraxane. The possible rationale for combining the checkpoint inhibitor with taxane-based chemotherapy that inhibits mitosis was that it may enhance tumor-antigen release and antitumor responses to immune checkpoint inhibition. Additionally, solvent-free taxanes might also activate toll-like receptors and promote dendritic cell activity (Figure 1) [45,46].
In the IMpassion130 trial, prior to treating patients with atezolizumab, tumor samples were centrally assessed by immunohistochemistry for the presence of PD-L1 expression (SP142 clone, Ventana). PD-L1 expression was assessed in tumor-infiltrating (immune) cells using two-tier system: “a percentage of tumor area” <1% (=PD-L1 negative) or ≥1% (=PD-L1 positive). The study revealed that the patients whose cancers were positive for PD-L1 (~41%) and received atezolizumab did significantly better than those treated with nab-paclitaxel: Median progression-free survival (PFS) was 7.2 months with atezolizumab compared with 5.5 months of the patients treated with placebo-nab-paclitaxel only. In the PD-L1-positive subgroup, the response rate was ~59% with atezolizumab–nab-paclitaxel in comparison with ~43% in the placebo–nab-paclitaxel subgroup. Notably, 10% of the patients in the atezolizumab–nab-paclitaxel group achieved a complete response as compared with only ~1% of those in the placebo–nab-paclitaxel group [44]. Importantly, the atezolizumab plus nab-paclitaxel combination group yielded no new safety concerns as the safety profile appeared consistent with the known profiles of each component drug. Indeed, the most common side effects with the combined treatment were hair loss, fatigue, tingling or numbness in the hands and feet, nausea and vomiting, diarrhea, anemia, constipation, cough, headache, neutropenia, and reduced appetite, which were present among 20% or more of patients.
One of the important findings in the IMpassion130 trial is the efficiency of combined therapy (immunotherapy + conventional chemotherapy). The combined treatment has its rationale at the molecular level as illustrated in the case of breast cancer in Figure 1. This approach has also been clinically verified to be successful in other cancers like NSCLC. Thus, the FDA in September 2017 approved the first combination of chemotherapy and immunotherapy for patients with metastatic, non-squamous NSCLC. The approval included pembrolizumab (KEYTRUDAÒ) in combination with pemetrexed and carboplatin. The approval was based on results obtained in the KEYNOTE-021 Phase 2 clinical trial that demonstrated that non-squamous NSCLC patients treated with the combination therapy exhibited a 55% therapeutic response rate in comparison with 29% with chemotherapy alone [47]. A recent update on this study (24 months follow-up) confirmed the initial data with significant improvements in PFS and objective response rate (~57% in combined group vs. 30% in chemotherapy group) in the patients treated with combined immunotherapy (pembrolizumab)-chemotherapy (pemetrexed-carboplatin) [48]. More recently, the FDA on March 18, 2019 has granted accelerated approval for the use of atezolizumab in combination with carboplatin and etoposide chemotherapy for the first-line treatment of adult patients with extensive-stage SCLC (ES-SCLC). Approval was based on the IMpower133 (NCT02763579), a randomized (1:1), multicenter, double-blind, placebo-controlled trial in 403 patients with ES-SCLC. Patients receiving atezolizumab with chemotherapy showed improved median overall survival of 12.3 months compared to 10.3 months for those on placebo with chemotherapy (hazard ratio [HR] 0.70; 95% CI: 0.54, 0.91; p = 0.0069). Furthermore, patients on the checkpoint inhibitor plus chemotherapy had a median PFS of 5.2 months compared with 4.3 months in the placebo with chemotherapy arm of the trial (HR 0.77; 0.62, 0.96; p = 0.0170) [49]. This approval has changed the standard first-line therapy for the first time in several decades for this cancer [50]. Despite concerns over the cost-effectiveness of checkpoint inhibitor-based immunotherapy [51], these recent FDA approvals (e.g., for NSCLC, ES-SCLC, and TNBC as discussed herein) have firmly established the use of checkpoint inhibitors together with conventional chemotherapy as a novel clinical paradigm for the treatment of otherwise difficult-to-treat cancers.
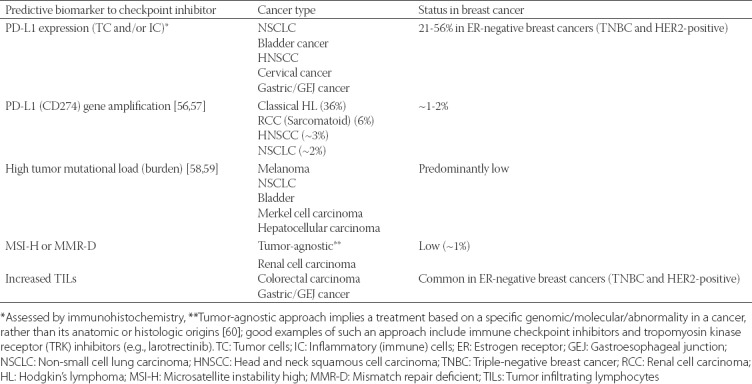
It is clinically important to identify patients who are likely to respond to checkpoint inhibitor-based immunotherapy. Although a quite difficult task given the complex tumor microenvironment and interactions between the cancer and various immune cells such as T-lymphocytes, B-lymphocytes, dendritic, and antigen-presenting cells (Figure 1), several biomarkers have nonetheless been identified to reliably predict the effectiveness of the anti-PD-1/PD-L1 checkpoint inhibitors in cancer patients. These include PD-L1 status (the target protein/antigen for atezolizumab), tumor mutational burden (load) [TMB/TML], microsatellite instability status (MSI) and the number of TILs (Tables 1 and 2). PD-L1 expression in cancer or immune cells as detected by immunohistochemistry has been shown to be one of the most reliable predictive biomarkers as confirmed in the IMpassion130 (NCT02425891) study. For detection purposes, four out of five diagnostic antibodies have been validated as either companion or complementary diagnostics (Table 1). Indeed, the current FDA approval of atezolizumab in the treatment of TNBC applies only to patients whose breast cancers express PD-L1 in an FDA-approved test (i.e., the Ventana diagnostic antibody SP142).
TABLE 2.
The status of predictive biomarkers to immune checkpoint inhibitors in cancers with approved anti-PD-1/PD-L1 agents and breast cancer
This recent FDA approval of atezolizumab plus chemotherapy for the treatment of adults with PD-L1-positive, unresectable, locally advanced, or metastatic TNBC represents the first cancer immunotherapy regimen to be approved for the management of breast cancer. It is truly a landmark therapeutic development for patients with TNBC given the limited treatment options available for this heterogeneous, but a highly aggressive subtype of breast cancer [52]. Chemotherapy alone had been the mainstay of treatment for many years for TNBC and so the approval of this checkpoint inhibitor combination for people with PD-L1-positive TNBC disease fulfills an unmet medical need. Hopefully, several additional ongoing trials with immune checkpoint inhibitors other than atezolizumab [41] will endorse the results from the IMpassion130 (NCT02425891) study. Additional efforts are also required to optimize predictive biomarkers in TNBC (PD-L1 antibody selection, Table 1, threshold for positivity, cancer vs. immune cells expression, further dissection of the breast cancer immune microenvironment, Figure 1) [53] and to maximize the effectiveness of these important class of immune-targeting therapeutic agents.
DECLARATION OF INTERESTS
ZG is employed by Caris Life Sciences, which commercially offers testing for PD-L1. Other authors declare no conflict of interests.
REFERENCES
- 1.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. https://doi.org/10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danilova L, Wang H, Sunshine J, Kaunitz GJ, Cottrell TR, Xu H, et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A. 2016;113(48):E7769–77. doi: 10.1073/pnas.1607836113. https://doi.org/10.1073/pnas.1607836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma:Implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244. https://doi.org/10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi: 10.1158/1078-0432.CCR-13-3271. https://doi.org/10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swoboda A, Nanda R. Immune checkpoint blockade for breast cancer. Cancer Treat Res. 2018;173:155–65. doi: 10.1007/978-3-319-70197-4_10. https://doi.org/10.1007/978-3-319-70197-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86. doi: 10.1158/2159-8290.CD-18-0367. https://doi.org/10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–52. doi: 10.1016/j.immuni.2018.03.014. https://doi.org/10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. https://doi.org/10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi: 10.1126/science.aan6733. https://doi.org/10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vranic S. Microsatellite instability status predicts response to anti-PD-1/PD-L1 therapy regardless the histotype:A comment on recent advances. Bosn J Basic Med Sci. 2017;17(3):274–5. doi: 10.17305/bjbms.2017.2366. https://doi.org/10.17305/bjbms.2017.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A, Djamgoz MB. Triple negative breast cancer:Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–22. doi: 10.1016/j.ctrv.2017.11.003. https://doi.org/10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Solinas C, Gombos A, Latifyan S, Piccart-Gebhart M, Kok M, Buisseret L, et al. Targeting immune checkpoints in breast cancer:An update of early results. ESMO Open. 2017;2(5):e000255. doi: 10.1136/esmoopen-2017-000255. https://doi.org/10.1136/esmoopen-2017-000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joneja U, Vranic S, Swensen J, Feldman R, Chen W, Kimbrough J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J Clin Pathol. 2017;70(3):255–9. doi: 10.1136/jclinpath-2016-203874. https://doi.org/10.1136/jclinpath-2016-203874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, et al. PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages:An assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol. 2017;41(3):334–42. doi: 10.1097/PAS.0000000000000780. https://doi.org/10.1097/PAS.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 15.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma:Correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–8. doi: 10.1593/neo.05733. https://doi.org/10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70. doi: 10.1158/2326-6066.CIR-13-0127. https://doi.org/10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett MT, Lenkiewicz E, Malasi S, Basu A, Yearley JH, Annamalai L, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20(1):71. doi: 10.1186/s13058-018-1004-0. https://doi.org/10.1186/s13058-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobral-Leite M, Van de Vijver K, Michaut M, van der Linden R, Hooijer GK, Horlings HM, et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology. 2018;7(12):e1509820. doi: 10.1080/2162402X.2018.1509820. https://doi.org/10.1080/2162402X.2018.1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovgaard ES, Dyhl-Polk A, Roslind A, Balslev E, Nielsen D. PD-L1 expression in breast cancer:Expression in subtypes and prognostic significance:A systematic review. Breast Cancer Res Treat. 2019;174(3):571–84. doi: 10.1007/s10549-019-05130-1. https://doi.org/10.1007/s10549-019-05130-1. [DOI] [PubMed] [Google Scholar]
- 20.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–70. doi: 10.1158/1055-9965.EPI-14-0654. https://doi.org/10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 21.Vranic S, Palazzo J, Sanati S, Florento E, Contreras E, Xiu J, et al. Potential novel therapy targets in neuroendocrine carcinomas of the breast. Clin Breast Cancer. 2019;19(2):131–6. doi: 10.1016/j.clbc.2018.09.001. https://doi.org/10.1016/j.clbc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Vranic S, Palazzo J, Swensen J, Xiu J, Florento E, Gatalica Z, et al. Theranostic molecular profiling of pleomorphic ductal carcinoma of the breast. Breast J. 2019;25(1):175–6. doi: 10.1111/tbj.13187. https://doi.org/10.1111/tbj.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vranic S, Feldman R, Gatalica Z. Apocrine carcinoma of the breast:A brief update on the molecular features and targetable biomarkers. Bosn J Basic Med Sci. 2017;17(1):9–11. doi: 10.17305/bjbms.2016.1811. https://doi.org/10.17305/bjbms.2016.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson ED, Taube JM, Asch-Kendrick RJ, Ogurtsova A, Xu H, Sharma R, et al. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod Pathol. 2017;30(11):1551–60. doi: 10.1038/modpathol.2017.79. https://doi.org/10.1038/modpathol.2017.79. [DOI] [PubMed] [Google Scholar]
- 25.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(1):52–63. doi: 10.1016/j.humpath.2015.09.003. https://doi.org/10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Huo L, Ma J, Zhao J, Bassett RL, Sun X, et al. Expression of programmed death ligand 1 (PD-L1) in posttreatment primary inflammatory breast cancers and clinical implications. Am J Clin Pathol. 2018;149(3):253–61. doi: 10.1093/ajcp/aqx162. https://doi.org/10.1093/ajcp/aqx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zawlik I, Gablo N, Szymanska B, Pawlowska Z, Chudobinski C, Chalubinska-Fendler J, et al. Immune checkpoints in aggressive breast cancer subtypes. Neoplasma. 2016;63(5):768–73. doi: 10.4149/neo_2016_514. https://doi.org/10.4149/neo_2016_514. [DOI] [PubMed] [Google Scholar]
- 28.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26(7):1488–93. doi: 10.1093/annonc/mdv192. https://doi.org/10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 29.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3(4):326–32. doi: 10.1158/2326-6066.CIR-14-0133. https://doi.org/10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altan M, Kidwell KM, Pelekanou V, Carvajal-Hausdorf DE, Schalper KA, Toki MI, et al. Association of B7-H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer. NPJ Breast Cancer. 2018;4:40. doi: 10.1038/s41523-018-0095-1. https://doi.org/10.1038/s41523-018-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tray N, Taff J, Singh B, Suh J, Ngo N, Kwa M, et al. Metaplastic breast cancers:Genomic profiling, mutational burden and tumor-infiltrating lymphocytes. Breast. 2019;44:29–32. doi: 10.1016/j.breast.2018.12.010. https://doi.org/10.1016/j.breast.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Barroso-Sousa R, Barry WT, Guo H, Dillon D, Tan YB, Fuhrman K, et al. The immune profile of small HER2-positive breast cancers:A secondary analysis from the APT trial. Ann Oncol. 2019 doi: 10.1093/annonc/mdz047. [Epub ahead of print] https://doi.org/10.1093/annonc/mdz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tawfik O, Kimler BF, Karnik T, Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018;80:170–8. doi: 10.1016/j.humpath.2018.06.008. https://doi.org/10.1016/j.humpath.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Pelekanou V, Barlow WE, Nahleh ZA, Wasserman B, Lo YC, von Wahlde MK, et al. Tumor-infiltrating lymphocytes and PD-L1 expression in pre- and posttreatment breast cancers in the SWOG S0800 phase II neoadjuvant chemotherapy trial. Mol Cancer Ther. 2018;17(6):1324–31. doi: 10.1158/1535-7163.MCT-17-1005. https://doi.org/10.1158/1535-7163.MCT-17-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z, et al. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer:The association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer. 2018;18(2):e237–44. doi: 10.1016/j.clbc.2017.11.001. https://doi.org/10.1016/j.clbc.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8(9):15584–92. doi: 10.18632/oncotarget.14698. https://doi.org/10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.AiErken N, Shi HJ, Zhou Y, Shao N, Zhang J, Shi Y, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13(9):1172–9. doi: 10.7150/ijbs.20868. https://doi.org/10.7150/ijbs.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis M, Tripathi S, Hughley R, He Q, Bae S, Karanam B, et al. AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PLoS One. 2018;13(6):e0196909. doi: 10.1371/journal.pone.0196909. https://doi.org/10.1371/journal.pone.0196909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1098–115. doi: 10.1158/2159-8290.CD-17-0222. https://doi.org/10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. Expression of PD-L1 and prognosis in breast cancer:A meta-analysis. Oncotarget. 2017;8(19):31347–54. doi: 10.18632/oncotarget.15532. https://doi.org/10.18632/oncotarget.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175–86. doi: 10.1016/S1470-2045(19)30026-9. https://doi.org/10.1016/S1470-2045(19)30026-9. [DOI] [PubMed] [Google Scholar]
- 42.Roche Registration Limited. Tecentriq (Atezolizumab):Summary of Product Characteristics. Welwyn Garden City, United Kingdom: Roche Registration Limited; 2018. [Google Scholar]
- 43.Genentech Inc. Tecentriq (Atezolizumab) South San Francisco, CA: Genentech Inc; 2018. [Google Scholar]
- 44.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. doi: 10.1056/NEJMoa1809615. https://doi.org/10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 45.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy:Harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–43. doi: 10.1158/2326-6066.CIR-15-0064. https://doi.org/10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yardley DA. Nab-paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–72. doi: 10.1016/j.jconrel.2013.05.041. https://doi.org/10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer:A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3. https://doi.org/10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. 24-month overall survival from KEYNOTE-021 cohort G:Pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung Cancer. J Thorac Oncol. 2019;14(1):124–9. doi: 10.1016/j.jtho.2018.08.004. https://doi.org/10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9. doi: 10.1056/NEJMoa1809064. https://doi.org/10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 50.Pacheco J, Bunn PA. Advancements in small-cell lung cancer:The changing landscape following IMpower-133. Clin Lung Cancer. 2019 doi: 10.1016/j.cllc.2018.12.019. [Epub ahead of print]. https://doi.org/10.1016/j.cllc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Zhou K, Zhou J, Huang J, Zhang N, Bai L, Yang Y, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of extensive-stage small-cell lung cancer. Lung Cancer. 2019;130:1–4. doi: 10.1016/j.lungcan.2019.01.019. https://doi.org/10.1016/j.lungcan.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Heimes AS, Schmidt M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs. 2019;28(1):1–5. doi: 10.1080/13543784.2019.1552255. https://doi.org/10.1080/13543784.2019.1552255. [DOI] [PubMed] [Google Scholar]
- 53.Gruosso T, Gigoux M, Manem VS, Bertos N, Zuo D, Perlitch I, et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. 2019;129(4):1785–800. doi: 10.1172/JCI96313. https://doi.org/10.1172/JCI96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsao MS, Kerr K, Dacic S, Yatabe Y, Hirsch FR. IASLC Atlas of PD-L1 Immunohistochemistry Testing in Lung Cancer. Aurora, Colorado: Editorial Rx Press; 2017. [Google Scholar]
- 55.Scheerens H, Malong A, Bassett K, Boyd Z, Gupta V, Harris J, et al. Current status of companion and complementary diagnostics:Strategic considerations for development and launch. Clin Transl Sci. 2017;10(2):84–92. doi: 10.1111/cts.12455. https://doi.org/10.1111/cts.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018;4(9):1237–44. doi: 10.1001/jamaoncol.2018.1701. https://doi.org/10.1001/jamaoncol.2018.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690–7. doi: 10.1200/JCO.2016.66.4482. https://doi.org/10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. https://doi.org/10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Vanderbilt CM, Cotzia P, Arias-Stella JA, 3rd, Chang JC, Zehir A, et al. Next-generation sequencing-based assessment of JAK2, PD-L1, and PD-L2 copy number alterations at 9p24.1 in breast cancer:Potential implications for clinical management. J Mol Diagn. 2019;21(2):307–17. doi: 10.1016/j.jmoldx.2018.10.006. https://doi.org/10.1016/j.jmoldx.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flaherty KT, Le DT, Lemery S. Tissue-agnostic drug development. Am Soc Clin Oncol Educ Book. 2017;37:222–30. doi: 10.1200/EDBK_173855. https://doi.org/10.1200/EDBK_173855; https://doi.org/10.14694/EDBK_173855. [DOI] [PubMed] [Google Scholar]


