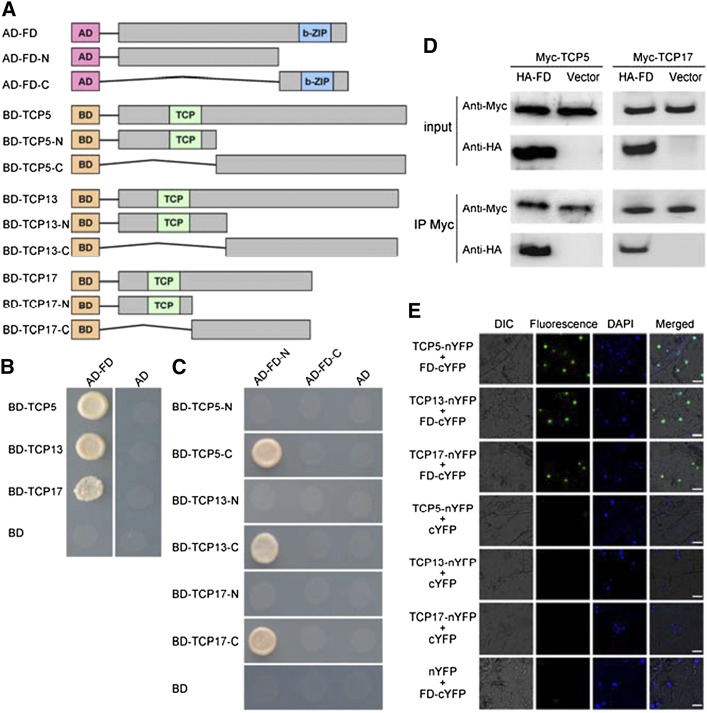
Figure 4.
Interaction between TCP5/13/17 and FD. A, Schematic representation of full-length and truncated FD and TCP5/13/17 constructs with specific deletions. B, Yeast two-hybrid assay analysis. Interaction was indicated by the ability of cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade. The GAL4 activation domain expressed by pGADT7 (shown as “AD”) was used as negative controls. The experiment was repeated three times with similar results and representative photos are displayed. C, C-termini of TCP5/13/17 and the N terminus of FD are required for their interactions. Interactions were indicated by the ability of yeast cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade. The GAL4 activation domain expressed by pGADT7 (shown as “AD”) was used as negative controls. The experiment was repeated three times with similar results and representative photos are displayed. D, Coimmunoprecipitation (CoIP) analysis. Myc-fused TCP5 and TCP17 were immunoprecipitated using anti-Myc antibody, and coimmunoprecipitated HA-FD was then detected using anti-HA antibody. Protein input for Myc-TCP5/17 and HA-FD in immunoprecipitated complexes were also detected and are shown. The experiment was repeated three times with similar results and representative photos are displayed. E, bimolecular fluorescence complementation (BiFC) analysis. Fluorescence was observed in nuclear compartments of N. benthamiana leaf epidermal cells; the fluorescence resulted from complementation of the C-terminal portion of yellow fluorescent protein (YFP) fused to FD (FD-cYFP) with the N-terminal portion of YFP fused to TCP5/13/17 (TCP5/13/17-nYFP). No signal was observed from negative controls. DAPI, 49,6-diamidino-2-phenylindole; DIC, Differential interference contrast. The experiment was repeated three times with similar results and representative photos are displayed. Scale bars = 25 μm.