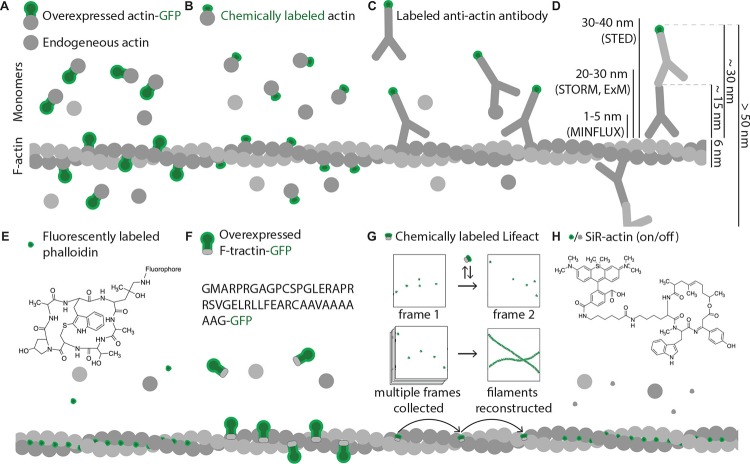
FIGURE 5.
Commonly used actin probes. Green shapes represent fluorescent moieties; all molecules shown in different shades of gray are non-fluorescent and hence are invisible under fluorescent microscope. Size differences of the shapes approximately represent size differences of the molecules. (A) Ectopically expressed actin-GFP partially incorporates into actin filaments but also contributes to background fluorescence from monomeric actin-GFP molecules and increases the concentration of the monomers. (B) Due to small size of dyes, chemically labeled actin has higher polymerization ability, however, still displays significant background fluorescence from monomers and also increases the concentration of actin monomers. (C) Density of antibody labeling depends on epitope accessibility and is significantly restricted by large size of antibody molecules, which also introduces large linkage errors; background fluorescence observed from antibodies bound to actin monomers in solution. (D) Comparison of fluorophore displacement from targeted epitope caused by common immunostaining procedures with resolution abilities of modern super resolution methods. When a combination of primary and secondary antibodies is used to image an actin filament, fluorophores of antibodies that recognize neighboring actin subunits might be located more than 50 nm apart (10 times larger distance then a thickness of an actin filament). Modern microscopy techniques can resolve objects that are as close as few nanometers apart, so usage of such large probes leads to significant loss of advantages super-resolution methods can offer. (E) Phalloidin is a small chemical that binds exclusively F-actin with high specificity and affinity, shows high density of labeling and low background signal. (F) Genetically encoded actin binders (F-tractin illustrated as an example) fused to GFP bind F-actin in vivo. Unbound molecules contribute to background fluorescence, but the concentration of actin monomers is not changed. (G) Low affinity of Lifeact binding to F-actin can be used for certain types of super resolution microscopy. Here Lifeact coupled to a dye acts as an exchangeable probe. Multiple frames are collected with Lifeact molecules having different locations in different frames. Post-imaging processing allows reconstructing F-actin architecture from all individual Lifeact locations. (H) SiR-actin is cell membrane-permeable, specifically labels F-actin and additionally has low fluorescence when not bound to F-actin (off state) but cannot be fixed by aldehydes.