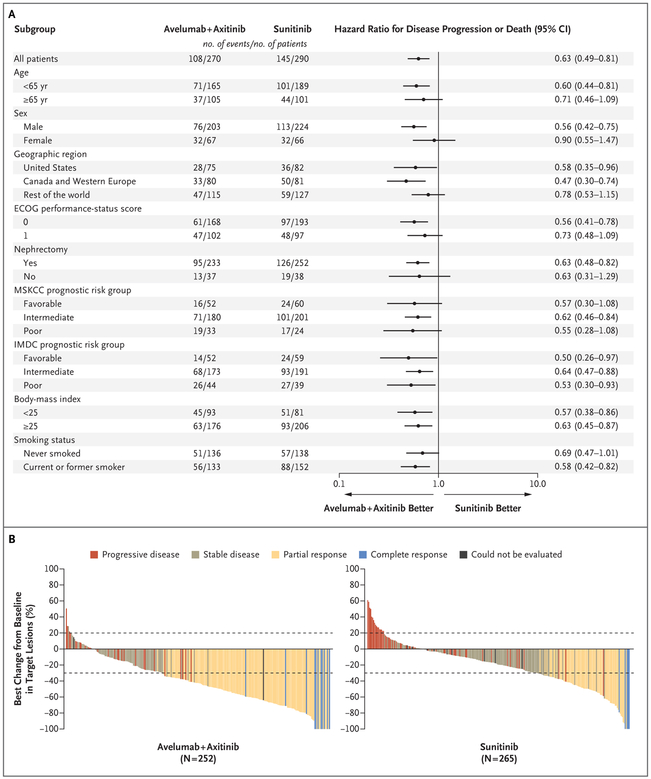
Figure 2. Subgroup Analyses of Progression-free Survival and Best Percentage Change in Target Lesions among Patients with PD-L1–Positive Tumors.
Panel A shows the results of a subgroup analysis of progression-free survival among the patients with PD-L1–positive tumors. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5,with higher numbers reflecting greater disability. Patients with favorable risk had a Memorial Sloan Kettering Cancer Center (MSKCC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 or more. MSKCC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80 (on a scale from 0 to 100, with lower scores indicating greater disability; patients with a performancestatus score of <70 were excluded from the trial), less than 1 year from the time of initial diagnosis to the start of therapy, a hemoglobin level below the lower limit of the normal range, a lactate dehydrogenase level more than 1.5 times the upper limit of the normal range, and a corrected serum calcium concentration of more than 10 mg per deciliter (2.5 mmol per liter). Patients with favorable risk had an International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score of 0, those with intermediate risk had a score of 1 or 2, and those with poor risk had a score of 3 to 6. IMDC risk scores are defined according to the number of the following risk factors present: a Karnofsky performance-status score of less than 80, time from initial diagnosis to randomization of less than 1 year, hemoglobin level below the lower limit of the normal range, corrected serum calcium level above the upper limit of the normal range, absolute neutrophil count above the upper limit of the normal range, and platelet count above the upper limit of the normal range. Body-mass index is the weight in kilograms divided by the square of the height in meters. Panel B shows the best percentage change from baseline in the sum of the longest diameters of target lesions in the patients with PD-L1–positive tumors. Dotted lines indicate Response Evaluation Criteria in Solid Tumors (RECIST)–defined progressive disease (≥20% increase in the sum of target-lesion diameters, with baseline as the reference) and partial response (≥30% decrease in the sum of target-lesion diameters, with baseline as the reference).