Abstract
Several studies have shown that statin users have a lower risk of new-onset dementia (NOD) compared nonusers. However, other studies have shown opposite results. In this study, we investigated the association between the use of statins and the development of NOD.
This was a longitudinal cohort study using data from claim forms submitted to the Taiwanese Bureau of National Health Insurance. The study included patients with NOD and non-NOD subjects from January 2002 to December 2013. We estimated the hazard ratios (HRs) of NOD associated with statin use, whereas nonuser subjects were used as a reference group.
A total of 19,522 NOD cases were identified in 100,610 hyperlipidemic patients during the study period. The risk of NOD, after adjusting for sex, age, comorbidities, and concurrent medication, was lower among statin users than nonusers (HR 0.95, 95% CI [confidence interval] 0.94–0.96; P < .001). The adjusted HRs for NOD were 1.53 (95% CI, 1.45–1.62), 0.63 (95% CI, 0.57–0.71), and 0.34 (95% CI, 0.30–0.38) when the cumulative defined daily doses ranged from 28 to 365, 366 to 730, and more than 730 relative to nonusers, respectively.
We concluded that statin use is associated with a decreased NOD risk. The protective effect of statins for NOD seemed to be related to high exposure to statins. This study also highlights that high exposure to statins has a dose-response effect on lowering NOD risk.
Keywords: high exposure to statins, hyperlipidemia, new-onset dementia
1. Introduction
The incidence of dementia is increasing worldwide, especially in the elderly population.[1] An estimated 46 million people with dementia were reported worldwide in 2015.[2] It is one of the most common causes of disability in this group.[3] Furthermore, dementia can increase healthcare costs because they adversely affect the patients’ quality of life.[4–6] Recent reports estimate that the expected economic burden is around 818 billion USD per year.[5] Therefore, concerns regarding medical care in dementia, particularly in terms of new-onset dementia (NOD), have increased.[7,8]
Statins are effective agents against hyperlipidemia and are widely used in the treatment of cerebrovascular or cardiovascular diseases.[9,10] In particular, statins may influence NOD risk by reducing beta-amyloid deposition.[11,12] Several randomized clinical trials have questioned whether the use of statins is associated with a lower risk of developing NOD in hyperlipidemic patients undergoing treatment.[13–18] However, the results of these studies are inconsistent, partly because of the insufficient follow-up periods and small sample size between statin users and nonusers.[14,19,20] Whether statins are associated with a lower risk of developing NOD when compared with nonuser patients with hyperlipidemia in a nationwide population during a long-term follow-up also remains unclear. Therefore, the objective of this study is to investigate the effects of statins on the risk of developing NOD among dyslipidemic patients in Taiwan.
2. Materials and methods
2.1. Study participants
Patients were included in the study if they had been diagnosed with hyperlipidemia and were statin users without dementia at baseline (January 1, 2002). We summarized the claim records of each patient into 1 record, and we used the International Classification of Diseases, Ninth Revision (ICD-9) Clinical Modification code to define dementia (ICD-9 codes 290). Statin users were those patients who received at least a 28-day statin prescription during the period between January 1 and December 31, 2002. In contrast, nonusers were those patients who did not receive a statin prescription throughout the whole study period.
2.2. Study design
Participants were included in the study if they had been newly diagnosed with hyperlipidemia between January 1 and June 30, 2002. At least 1 of the following enrollment criteria had to be met for inclusion in the study: 2 or more outpatient visits within a 6-month period, continuous administration of all statin prescriptions for more than 6 months within a 12-year follow-up period, or 1 or more inpatient admissions with a diagnosis of hyperlipidemia. The primary endpoint was the development of NODs, which was defined by the time dementia (ICD-9-CM codes 290) first appeared in the inpatient or outpatient claim records. Comorbidities related to dementia were defined according to the ICD-9-CM code and included coronary heart disease (CHD; ICD-9-CM code 410–415), hypertension (ICD-9-CM code 401–405), diabetes mellitus (DM) (ICD-9-CM code 250), and cerebrovascular attack (CVA; ICD-9-CM code 430–438). In a previous study, Zissimopoulos et al[18] defined high statin exposure as “at least the 50th percentile of days of filled prescriptions in a given year for at least 2 years during 2006, 2007, and 2008.” Therefore, defined daily doses (DDDs) >730 was considered as high exposure and ≤730 as low exposure. Patients who fulfilled any of the following criteria were excluded from the study: a prior history of dementia before January 1, 2002, and patients aged more than 85 years. Initially, 104,858 outpatients fulfilled the inclusion criteria; however, 1128 patients were excluded based on the diagnosis of dementia before January 1, 2002. A total of 103,730 patients were enrolled in the study at baseline (Fig. 1). Furthermore, 1851 patients who were lost to follow-up and 1269 patients who died without the diagnosis of NODs or NOD-related deaths were excluded. Finally, a total of 100,610 outpatients were involved in this study, and it included 38,012 patients with Alzheimer's disease.
Figure 1.
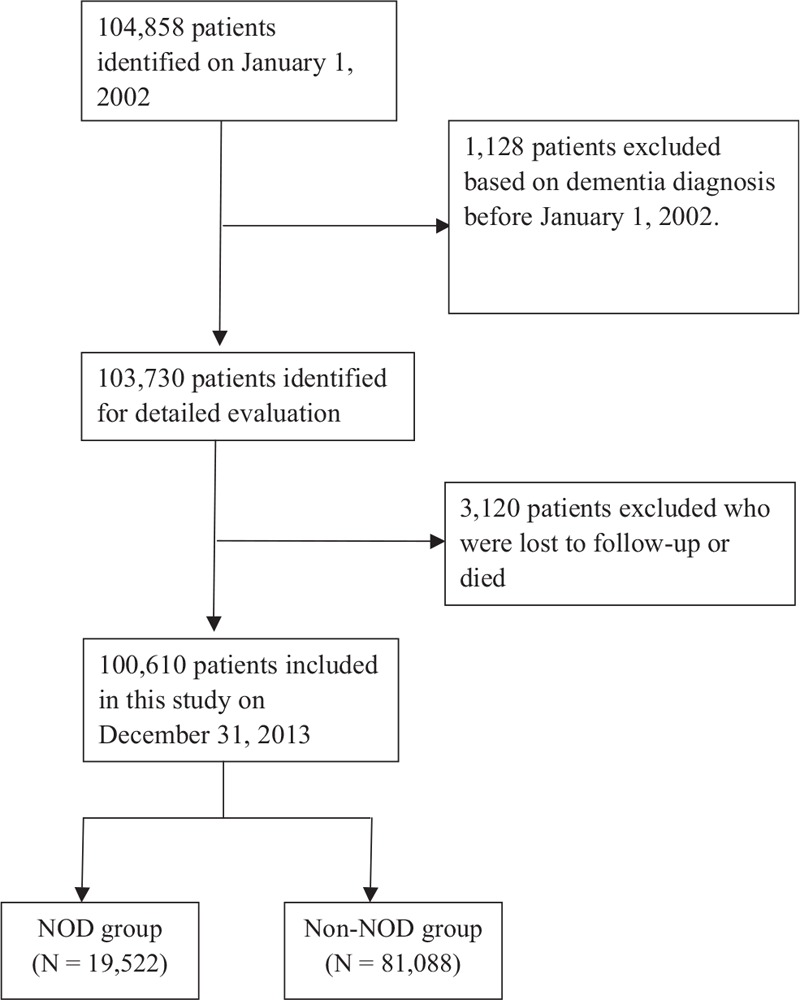
Flowchart of all patients for the inclusion in this study. NOD = new-onset dementia.
2.3. Ethical considerations
This study was approved by the ethics committee of the Chung Shan Medical University Hospital (CS2-17084). Written consent was not obtained from the study participants as only deidentified data were obtained from the longitudinal health insurance database 2010, and a waiver of patient consent was provided by the ethics committee for this study.
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation. Differences in continuous variables were analyzed using an unpaired Student t test. Categorical and discrete variables were presented as frequencies and percentages. The NOD-free survival rates in the 3 groups were calculated using the Kaplan–Meier method using the log-rank test. The Cox proportional hazard regression model was used to compare the risk of NOD development between statin users and nonusers. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, adjusting for important risk factors for the development of dementia that includes age, sex, medication use, and comorbidities. P < .05 were considered to be statistically significant. All statistical calculations were performed using statistical analysis software, version 9.3 (SAS Institute, Inc, Cary, NC).
3. Results
3.1. Study participants
Table 1 presents the baseline characteristics, medication use, and comorbidities among the studied population. Among the 100,610 eligible patients with defined hyperlipidemia, 19,522 (19.4%) developed NOD between January 2002 and December 2013. More women than men were observed in this study population. The mean age of patients with NOD was 74.0 years, and that of non-NOD patients was 71.1 years, indicating a statistically significantly difference (P < .001).
Table 1.
Baseline characteristics of all patients.
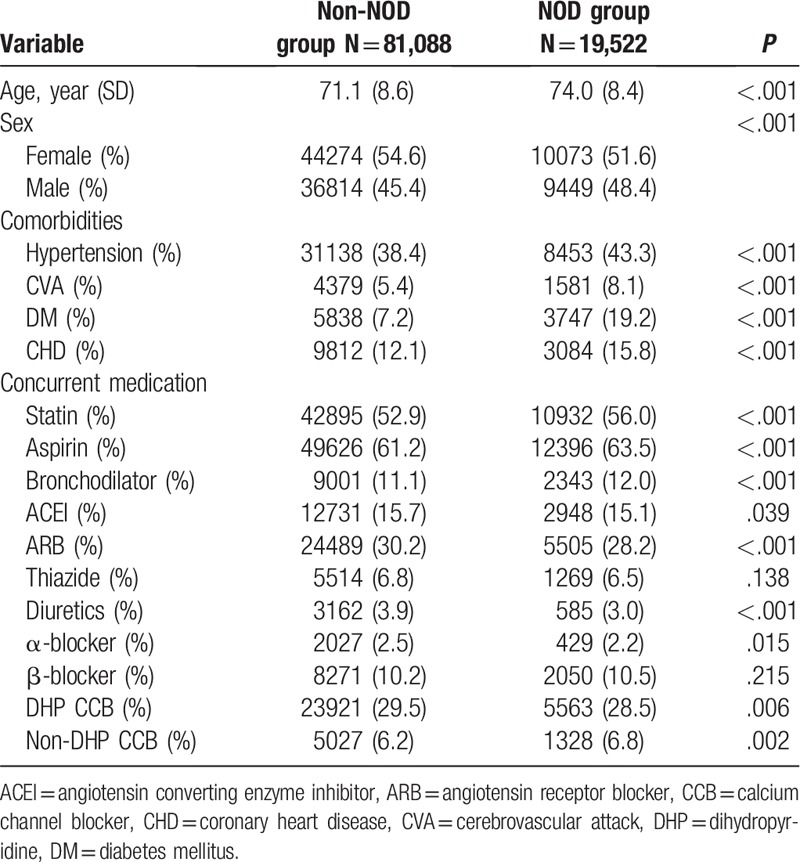
Patients in the NOD group had more comorbidities, including hypertension, CVA, DM, and CHD. There was a significantly higher percentage of statin users in the NOD group compared to the non-NOD group (56.0 vs. 52.9, P < .001).
Regarding the concurrent medication used at the beginning of the study, aspirin was the most prescribed medication and was used by 63.5% of patients in the NOD group and 61.2% of patients in the non-NOD group. The use of bronchodilators, beta-blockers, and nondihydropyridine calcium channel blockers was also slightly higher in the NOD group compared to the non-NOD group. Conversely, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide, diuretics, α-blocker, and dihydropyridine calcium channel blockers were less prescribed in the NOD group compared to the non-NOD group.
3.2. The relative risk of NOD
The result of the Kaplan–Meier survival analysis is shown in Figure 2. Our findings indicate that the risk of developing NODs was significantly lower among statin users than nonusers (P < .001).
Figure 2.
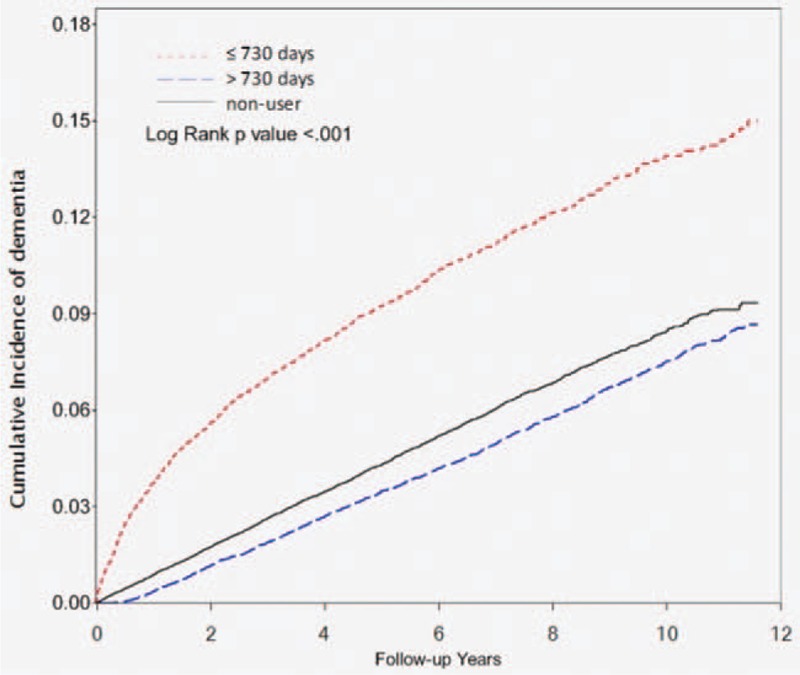
Kaplan–Meier analysis comparing probabilities of new-onset dementia between patients with statin user and nonuser.
The analyses using DDDs indicated a tendency of decreasing risk of NODs with increasing DDDs. The high DDDs (>730) of statins showed a significantly lower risk of NOD development (crude HR, 0.46; 95% CI, 0.42–0.50; adjusted HR, 0.44; 95% CI, 0.40–0.49). Conversely, the low DDDs (28–365 and 366–730) of statins showed a significant association with the risk of NOD development (crude HRs, 1.75 and 1.10; 95% CIs, 1.66–1.86 and 0.99–1.21; adjusted HRs, 1.90 and 1.13; 95% CIs, 1.79–2.01 and 1.02–1.24, respectively).
Patients who took statins (crude HR, 0.94; 95% CI, 0.94–0.95; adjusted HR, 0.95; 95% CI, 0.94–0.96) were at lower risk of developing NODs compared with nonusers. The crude HR of developing NODs was lower among nonusers compared with simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, and rosuvastatin users (P < .05; Table 2). However, after adjusting for age, sex, comorbidities, and concurrent medication use, all statins were not associated with the risk of developing NODs compared with nonusers (P > .05; Table 2). Similarly, lipophilic and hydrophilic statins showed similar effects in patients with hyperlipidemia in terms of NOD development risk (crude HRs, 0.93 and 0.95; 95% CIs, 0.91–0.95 and 0.93–0.97; adjusted HRs, 0.94 and 0.96; 95% CIs, 0.92–0.96 and 0.93–0.99, respectively).
Table 2.
Crude and adjusted hazards ratios of statins for new-onset dementia.
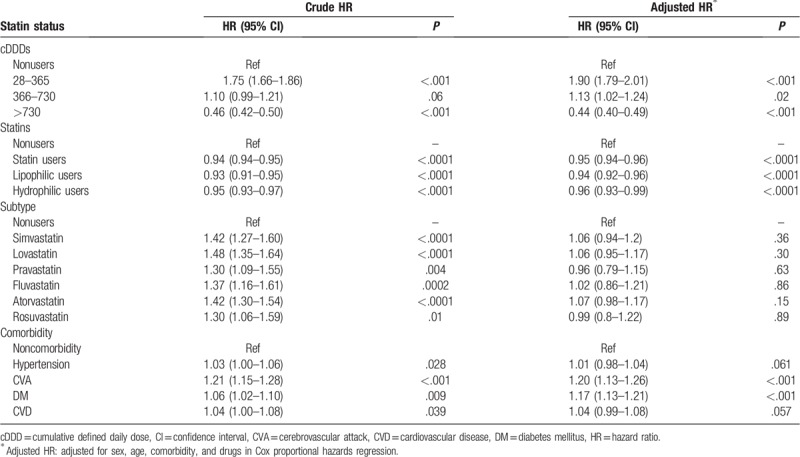
The crude HR of developing NODs was higher among comorbidities for hypertension and CHD (HRs, 1.03 and 1.04; 95% CIs, 1.00–1.06 and 1.00–1.08, respectively; Table 2). However, after adjusting for age, sex, comorbidities, and concurrent medication use, the risk for NOD development was not associated with comorbidities for hypertension and CHD (HRs, 1.01 and 1.04; 95% CIs, 0.98–1.08 and 0.99–1.08, respectively; Table 2). Patients with comorbidities for CVA (crude HR, 1.21; 95% CI, 1.15–1.28; adjusted HR, 1.20; 95% CI, 1.13–1.26) and DM (crude HR, 1.06; 95% CI, 1.02–1.10; adjusted HR, 1.17; 95% CI, 1.13–1.21) were at higher risk of developing NODs compared with non-CVA and non-DM patients.
4. Discussion
This study indicated that the risk of developing NODs was significantly lower in statin users than nonusers. This finding suggests that continuously using a statin could have a potential impact in preventing the development of NODs. This study also demonstrated that patients with comorbidities for CVA and DM were at higher risk of developing NODs compared with those with non-CVA and non-DM. The risk for NOD development was not associated with comorbidities for hypertension and CHD in patients with hyperlipidemia. Finally, our study also demonstrated that high exposure to statins has a dose–response effect on lowering the NOD risk.
Several observational studies have shown that statin use has a positive effect on emerging dementia.[16–21] However, randomized clinical trials have not drawn sufficient conclusions that the risk of developing NODs was significantly lower in statin users than nonusers because of short follow-up time, small sample size, and the absence of dyslipidemic participants.[15,16,22] Previous observational studies have also obtained similar results to our study,[18] where they evaluated 399,979 patients and found that statin use was associated with a reduced risk of Alzheimer's disease in white women, white men, Hispanic women, Hispanic men, and black women. In that study, only statin use was not associated with a reduced risk of Alzheimer's disease among black man. Conversely, Roy et al[22] found that statin was significantly associated with an increased risk of NOD development. In their study, they observed that dementia was an associated comorbid condition in 7.9% of patients using statins compared with 3.1% of statin nonusers. It is important to mention that many observational studies found no association between statins and dementia.[23–25]
In the present study, patients using high DDDs (>730) of statin have a significant protective effect against NODs, whereas low DDDs of statin were significantly associated with a higher risk of developing NODs. This demonstrates that statins have a dose–response effect on lowering the NOD risk. Zissimopoulos et al[33] studied individuals categorized as having high statin exposure, which fell into at least the 50th percentile of days of filled prescriptions for at least 2 out of the last 3 years. Users not meeting these characteristics were categorized as having low statin exposure. According to this rule, DDDs >730 are considered as higher exposure and ≤730 as low exposure. Higher exposure to statins seemed to be particularly effective in preventing dementia. To the best of our knowledge, there are a few studies that directly compare the incidence of dementia in patients treated with statins during a long-term follow-up. All the previous NOD studies were placebo-controlled and had a short duration (2–5 years).[19,20] Furthermore, these observational studies did not use a DDD for continuous prescription of statins. However, we are not certain whether our findings are consistent with those of previous observational studies. Additional studies using a DDD for continuous statins prescription are required in the future.
The present study showed that the associations varied according to the dose of statin. The reasons for these findings in our study are unclear, but they may be attributable to the statin dose (high vs. low) and duration (>2 vs. ≤2 years).[18–20] These findings emphasize the need for further investigation of the mechanistic links between higher statin exposure and NOD.
In our study, there was a significantly higher association with NOD in patients with CVA and DM compared with those with non-CVA and non-DM. This result is consistent with the results reported by Roy et al,[22] who showed a statistically significant high risk of developing NODs (3.2 times greater odds in CVA and 1.6 times greater odds in DM). Conversely, the patients with hypertension and CHD were not associated with the NOD risk. Our findings do not fully correlate with the previously reported studies.[26,27] Moreover, the mechanisms of the findings in our study are unclear. Further prospectively randomized studies are needed to confirm our findings.
In vitro data show that serum cholesterol levels are linked to beta-amyloid deposition, Alzheimer's disease, and dementia pathology.[11,20,28–31] Therefore, the role of cholesterol in beta-amyloid processing has led to the hypothesis that cholesterol-moderating drugs could influence the onset and progression of dementia. Statins are hydrophilic or lipophilic, and it is generally considered that lipophilic statins cross the blood-brain barrier faster, leading to believe that they could decrease the risk of developing NODs faster than hydrophilic statins.[16,32] However, in our study that included a 12-year follow-up period, the association with NOD risk reduction was similar between lipophilic and hydrophilic statins. Similarly, our results are consistent to that reported by Ward et al[33] who enrolled 2003 pairs of statin users and nonusers who were elderly patients and showed a reduction in the risk of developing NODs in statin users compared with statin nonusers.[33] In particular, a risk reduction among the users that had a high dose–response effect (high exposure to statins) on NOD risk was observed in our study. The reason for these findings in our study is unclear, but it may be attributable to the statin dose (high vs. low).[18,33,34] These findings emphasize the need for further randomized control trials that ascertain our results.
Some limitations of this study need to be emphasized. First, all cases in this study were collected from claimed datasets of the Taiwan National Health Insurance that the diagnoses were based on physician reports only; therefore, it is unclear how our findings can be generalized to patients in different areas of the world. Second, this was a descriptive retrospective study conducted in Taiwan over a period of 12 years. Moreover, we excluded irregularly treated dyslipidemic patients from the analyses. Therefore, caution must be exercised in interpreting our data. Third, the process of dementia in patients who developed NOD in this study would have started many years before the diagnoses, and NOD may have coexisted with the process of hyperlipidemia for which statins were used. Thus, the cause-and-effect relationships between NOD and statins cannot be determined in this study.
5. Conclusion
Our results show that statin use may be associated with a decreased risk of NOD. The protective effect of statins against NOD seemed to be more prominent with high exposure to statins. This study also highlighted that high exposure to statins has a dose–response effect on lowering NOD risk. Further prospective randomized studies are needed to confirm our findings.
Author contributions
Conceptualization: Chih-Feng Chang, Yi-Sheng Liou, Tsung-Kun Lin, Hung-Yi Chen, Gwo-Ping Jong.
Funding acquisition: Chih-Feng Chang, Tsung-Kun Lin, Hung-Yi Chen.
Investigation: Chih-Feng Chang, Yi-Sheng Liou, Tsung-Kun Lin, Stacey Ma, Yu-Ru Hu.
Methodology: Chih-Feng Chang, Yi-Sheng Liou, Hung-Yi Chen, Gwo-Ping Jong.
Project administration: Chih-Feng Chang, Yi-Sheng Liou, Stacey Ma, Yu-Ru Hu, Gwo-Ping Jong.
Resources: Chih-Feng Chang, Yi-Sheng Liou, Tsung-Kun Lin.
Supervision: Yi-Sheng Liou, Hung-Yi Chen, Gwo-Ping Jong.
Visualization: Chih-Feng Chang, Yi-Sheng Liou.
Writing – original draft: Chih-Feng Chang, Gwo-Ping Jong.
Writing – review & editing: Hung-Yi Chen, Gwo-Ping Jong.
Gwo-Ping Jong orcid: 8889-8889-8889-888X.
Footnotes
Abbreviations: ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker, CCB = calcium channel blocker, cDDD = cumulative defined daily dose, CHD = coronary heart disease, CI = confidence interval, CVA = cerebrovascular attack, CVD = cardiovascular disease, DDD = defined daily dose, DHP = dihydropyridine, DM = diabetes mellitus HR = hazard ratio, ICD-9 = International Classification of Diseases, Ninth Revision, NOD = new-onset dementia.
C-FC and Y-SL contributed equally to this work; H-YC and G-PJ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med 2013;369:2275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].GBD 2015 Disease, Injury Incidence, Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burns A, Iliffe S. Dementia. BMJ 2009;338:b75. [DOI] [PubMed] [Google Scholar]
- [4].Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 2011;7:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prince M, Wimo A, Guerchet M, et al. World Alzheimer report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- [6].Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [7].Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. [DOI] [PubMed] [Google Scholar]
- [8].Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer's disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health 2015;105:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 2013;6:390–9. [DOI] [PubMed] [Google Scholar]
- [11].Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 2001;8:890–9. [DOI] [PubMed] [Google Scholar]
- [12].Di Paolo G, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci 2011;12:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McGuinness B, Passmore P. Can statins prevent or help treat Alzheimer's disease? J Alzheimers Dis 2010;20:925–33. [DOI] [PubMed] [Google Scholar]
- [15].Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol 2011;68:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Corrao G, Ibrahim B, Nicotra F, et al. Long-term use of statins reduces the risk of hospitalization for dementia. Atherosclerosis 2013;230:171–6. [DOI] [PubMed] [Google Scholar]
- [17].Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet 2000;356:1627–31. [DOI] [PubMed] [Google Scholar]
- [18].Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol 2017;74:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity: the Rotterdam Study. J Neurol Neurosurg Psychiatry 2009;80:13–7. [DOI] [PubMed] [Google Scholar]
- [20].Hendrie HC, Hake A, Lane K, et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn Dis 2015;25:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong W, Lin V, Boudreau D, et al. Statins in the prevention of dementia and Alzheimer's disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf 2013;22:345–58. [DOI] [PubMed] [Google Scholar]
- [22].Roy S, Weinstock JL, Ishino AS, et al. Association of cognitive impairment in patients on 3-hydroxy-3-methyl-glutaryl-coA reductase inhibitors. J Clin Med Res 2017;9:638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006;97(8A):52C–60C. [DOI] [PubMed] [Google Scholar]
- [24].Rea T, Breitner J, Psaty B, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 2005;62:1047–51. [DOI] [PubMed] [Google Scholar]
- [25].Rojas-Fernandez C, Hudani Z, Bittner V. Statins and cognitive side effects: what cardiologists need to know. Cardiol Clin 2015;33:245–56. [DOI] [PubMed] [Google Scholar]
- [26].Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med 2014;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010;7:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Petanceska SS, DeRosa S, Olm V, et al. Statin therapy for Alzheimer's disease: will it work? J Mol Neurosci 2002;19:155–61. [DOI] [PubMed] [Google Scholar]
- [29].Lesser GT, Beeri MS, Schmeidler J, et al. Cholesterol and LDL relate to neuritic plaques and to APOE4 presence but not to neurofibrillary tangles. Curr Alzheimer Res 2011;8:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kandiah N, Feldman HH. Therapeutic potential of statins in Alzheimer's disease. J Neurol Sci 2009;283:230–4. [DOI] [PubMed] [Google Scholar]
- [31].Reed B, Villeneuve S, Mack W, et al. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 2014;71:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vuletic S, Riekse RG, Marcovina SM, et al. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord 2006;22:392–8. [DOI] [PubMed] [Google Scholar]
- [33].Wu CK, Yang YH, Lin TT, et al. Statin use reduces the risk of dementia in elderly patients: a nationwide data survey and propensity analysis. J Int Med 2015;277:343–52. [DOI] [PubMed] [Google Scholar]
- [34].Chou CY, Chou YC, Chou YJ, et al. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol 2014;173:305–10. [DOI] [PubMed] [Google Scholar]


