Abstract
BACKGROUND: Seventy percent of intrahepatic cholangiocarcinoma (ICC) patients are inoperable. Treatment for unresectable patients is essential to improve poor survival. AIMS: We aimed to evaluate the prognostic factors for ICC patients, and investigate the potential treatment strategies for unresectable patients. METHODS: ICC patients were identified in SEER registry in 2004–2013. Univariate and multivariate Cox proportional hazard regression analysis were performed to evaluate the effect of treatment strategies. RESULTS: Of 2248 cases diagnosed in 2010–2013 and staged according to the American Joint Committee on Cancer (AJCC) 7th edition, 1706 (76.13%) did not receive cancer-directed surgery. This portion increased compared to those diagnosed between 2004 and 2009 and staged according to the AJCC 6th edition (72.87%). In addition, the percentage of stage 4 cases increased, while stage 3 cases decreased, because AJCC 7th staging system categorized both T4 and N1 patients into stage IV, which were previously categorized into stage III by AJCC 6th staging system. Patients with radiofrequency ablation (RFA) showed a poorer survival in 2004–2009 (P = .0213), but an almost the same survival as patients with tumor resection in 2010–2013 (P = .51), suggesting that RFA performed better in recent years. Lymphadenectomy showed protective effect for unresectable patients. Radiotherapy improved cancer-specific survival in non-surgery patients (P < .0001).The proportion of stage IV patients increased tremendously from 37.4% in 2004–2009 to 58.7% in 2010–2013. Among 1319 stage IV patients (2010–2013), surgery at distant metastatic sites improved cancer-specific survival. CONCLUSIONS: For unresectable tumors, RFA, radiotherapy, lymphadenectomy, and surgery of distant metastases showed significant benefits to improve cancer-specific survival.
Background
Intrahepatic cholangiocarcinomas (ICC) are located within the hepatic parenchyma. The prognosis of ICC patients were poor. Tumor resection with negative margins provides the best chance for prolonged survival [1], whereas resection with margins appears to be superior to non-operative treatment [2]. According to NCCN guidelines, multifocal hepatic diseases, lymph node metastases beyond the porta hepatis and distant metastatic diseases contraindicate tumor resection [3], [4]. Tumor resection is only considered in highly selected cases with limited multifocal diseases or gross lymph node metastases to the porta hepatis [5], [6]. To improve the overall survival outcomes, many other strategies have been developed for unresectable ICC patients.
For unresectable ICC patients, heat-RFA is supposed to be a safe and effective treatment [7]. It successfully controls local primary tumors of intermediate (3–5 cm) or small (<3 cm) diameter and resulted in a median overall survival of 38.5 months [8]. Studies on SEER data between 1983 and 2010 suggest an increasing use of RFA and surgical resection and a decreasing use of radiation alone [9]. Radiation is another option for unresectable ICC patients. Radiotherapy exhibits benefits on overall survival among non-surgery ICC patients who receive chemotherapy [10]. Postoperative intensity-modulated radiotherapy can improve overall survival and disease-free survival in ICC abutting vasculature with null-margin resection [11]. ICC has a high tendency toward metastasis; lymphadenectomy is likely to have a significant impact on the overall management of ICC. Lymphadenectomy has shown a survival benefit and is thus suggested to be considered for all patients [12].
In this study, we aimed to investigate the risk factors of mortality and evaluate the survival benefit of potential treatment strategies (RFA, radiotherapy and lymphadenectomy) for unresectable ICC patients by using SEER database from 2004 to 2013.
Methods
Database and Cohort Definition
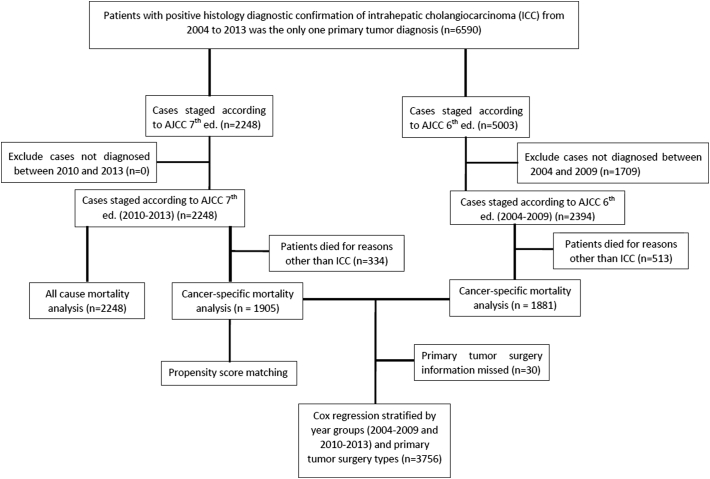
A total of 12,780 patients diagnosed with ICC (ICD-O-3 Histology/behavior code: 8160/3; site recode ICD-O-3/WHO 2008 code: intrahepatic bile duct and liver) were identified in the SEER 18 Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying) incidence database. As shown in the flow diagram in Figure 1, we included 2394 histologically confirmed cases diagnosed from 2004 to 2009 and staged by the AJCC 6thedition TNM staging system and 2248 histologically confirmed cases diagnosed from 2010 to 2013 and staged by the AJCC 7thedition TNM staging system. Demographics, AJCC TNM stages, tumor differentiation grade, and treatment information were compared.
Figure 1.
Flow diagram.
Treatment Categories
SEER data were collected and reported using data items and codes as documented by the North American Association of Central Cancer Registry (NAACCR) [13]. All patients included were divided into 2004–2009 subgroup and 2010–2013 subgroup, according to the different AJCC editions of the staging system. According to AJCC TMN Staging (7th ed., 2010), all tumors with periductal invasion (T4), lymph node metastases (N1) or distant metastasis (M1) are categorized as stage IV. But, according to AJCC TMN Staging (6th ed.), only tumors with M1 are categorized as stage IV. For stage IV patients, ICC tumors were unresectable, so other treatment strategies than tumor resection were important to improve survival.
Based on information regarding primary cancer-directed surgery types, all recruited patients were categorized into a non-cancer-directed surgery group, a tumor resection group, an ablation (RFA) group and a tumor destruction (other than ablation) group. Based on the radiotherapy information, regional lymph node (LN) surgery and distant site surgery, patients were also divided into radiation / non-radiation group, lymphadenectomy/non-lymphadenectomy group, and distant metastatic site surgery / non-distant metastatic site surgery group. Radiotherapy included beam radiation, radioactive implants, radioisotopes, and combinations of two or three of the radiotherapy types. Lymphadenectomy was the regional lymph node(s) removal surgery type, and distant metastatic site surgery was the non-primary surgical procedure for distant lymph node(s) and / or distant metastatic sites.
Statistical Analysis
Patients were followed up until December 2013. The primary outcomes measured were all-cause mortality and cancer-specific mortality. The candidate risk factors included age, sex, race, tumor differentiation grade, AJCC TMN stage, and treatment types. Numeric variables were summarized as the mean (standard deviation) and median (interquartile range). Categorical variables were reported as counts (percentage). An analysis of variance was used to compare continuous variables with symmetric distributions across the 2004–2009 and 2010–2013 subgroups. chi-square tests or Fisher's exact tests (n < 5) were used to compare categorical variables between subgroups. The Kaplan–Meier method was used to plot the survival distributions, and the log-rank test was used to assess differences in survival experience among the treatment subgroups. To identify the risk factors for cancer-specific mortality and all-cause mortality, univariate Cox proportional hazards regression was performed to estimate the hazard ratio. To further adjust for potential baseline confounders, multivariate Cox regression was carried out. In multivariate model, the confounders included age, sex, race, tumor grade, T-stage, N-stage, M-stage, AJCC stage, cancer-directed surgery, regional lymph node surgery, surgery at distant sites, and radiation. To evaluate the effect of radiation on survival for different subgroups by the stratification variables, stratified Cox regression models were performed. In stratified COX model, the effect of radiation was adjusted by the stratified variable and their interaction term (the effect of radiation was evaluated by multiplying with stratified variable). So, the P-value for interaction term indicated the different effect of radiation among the stratified subgroups. All tests of hypotheses were two-tailed and conducted at a significance level of 0.05. Statistical analyses were conducted using SAS 9.4.
Results
Demographic and Clinical Changes Between 2004 to 2009 and 2010 to 2013
Of 4642 cases included in this analysis, 2248 were diagnosed in 2010–2013 and staged by the AJCC 7th edition (group 1), and 2394 cases were diagnosed in 2004–2009 and staged by the AJCC 6th edition (group 2). The shorter follow-up time in group 1 resulted in a shorter survival in months, lower all-cause mortality and lower cancer-specific mortality rates than patients in group 2. Major T stage changes existed in the T2 and T3 categories. In the AJCC 6th edition, multiple tumors less than 5 cm were in T2, and multiple tumors more than 5 cm were in T3. However, in the AJCC 7th edition, the T2 stage included multiple tumors, ignoring tumor size. As shown in Table 1, patients diagnosed from 2010 to 2013 had many more individuals in T2 and stage II compared to patients diagnosed from 2004 to2009 patients. Still, unlike the AJCC 6th edition, which included T4 and N1 in stage III, the AJCC 7th edition categorized both T4 and N1 patients in stage IV. Thus, 58.67% patients diagnosed from 2010 to 2013 were in stage IV, and only 4.72% were in stage III. Moreover, compared to patients diagnosed from 2004 to 2009, more patients diagnosed from 2010 to 2013 had lymph node metastasis (P < .0001) and distant metastasis (P = .03).
Table 1.
Characteristics for patients staged by AJCC 6th and 7th edition
| Covariate | Level | AJCC 7TH 2010–2013 (n = 2248) | AJCC 6th 2004–2009 (n = 2394) | P-value |
|---|---|---|---|---|
| Age | 66.08 ± 12.61, 67 (58, 75) | 65.45 ± 13.08, 66 (56, 75) | .10 | |
| Sex | Male | 1132 (50.36%) | 1206 (50.38%) | .99 |
| Female | 1116 (49.64%) | 1188 (49.62%) | ||
| Survival months | 9.05 ± 10.11, 5 (2, 13) | 16.52 ± 23.39, 7 (2, 20) | <.0001 | |
| All-cause death | Alive | 765 (34.03%) | 216 (9.02%) | <.0001 |
| Death | 1483 (65.97%) | 2178 (90.98%) | ||
| Cancer-specific death | Alive | 765 (40.16%) | 216 (11.48%) | <.0001 |
| Death | 1140 (59.84%) | 1665 (88.52%) | ||
| Race | white | 1729 (76.91%) | 1856 (77.53%) | .78 |
| black | 180 (8.01%) | 193 (8.06%) | ||
| Chinese | 66 (2.94%) | 76 (3.17%) | ||
| Others | 273 (12.14%) | 269 (11.24%) | ||
| Grade | Well-differentiated | 99 (10.43%) | 129 (11.46%) | .29 |
| Moderate | 453 (47.73%) | 530 (47.07%) | ||
| Poorly differentiated | 388 (40.89%) | 446 (39.61%) | ||
| undifferentiated | 9 (0.95%) | 21 (1.87%) | ||
| T stage | T0 | 8 (0.40%) | 7 (0.34%) | <.0001 |
| T1 | 669 (33.62%) | 841 (41.37%) | ||
| T2 | 840 (42.21%) | 308 (15.15%) | ||
| T3 | 279 (14.02%) | 560 (27.55%) | ||
| T4 | 194 (9.75%) | 317 (15.59%) | ||
| N stage | N0 | 1419 (69.42%) | 1653 (76.46%) | <.0001 |
| N1 | 625 (30.58%) | 509 (23.54%) | ||
| M stage | M0 | 1338 (59.52%) | 1499 (62.61%) | .03 |
| M1 | 910 (40.48%) | 895 (37.39%) | ||
| AJCC stage | I | 440 (19.57%) | 590 (24.64%) | <.0001 |
| II | 383 (17.04%) | 195 (8.15%) | ||
| III | 106 (4.72%) | 714 (29.82%) | ||
| IV | 1319 (58.67%) | 895 (37.39%) | ||
| Cancer-directed surgery | None | 1706 (76.13%) | 1735 (72.87%) | .01 |
| Surgery, NOS∗ | 5 (0.22%) | 11 (0.46%) | ||
| Resection | 484 (21.60%) | 574 (24.11%) | ||
| Ablation | 35 (1.56%) | 33 (1.39%) | ||
| Tumor destruction∗∗ | 11 (0.49%) | 28 (1.18%) | ||
| Regional lymph node surgery | None | 1866 (85.87%) | 1991 (84.69%) | .26 |
| Yes | 307 (14.13%) | 360 (15.31%) | ||
| Surgery at distant sites | No | 2139 (98.03%) | 2265 (97.97%) | .88 |
| Yes | 43 (1.97%) | 47 (2.03%) | ||
| Radiation | None | 1916 (86.31%) | 2048 (86.49%) | .86 |
| Performed | 304 (13.69%) | 320 (13.51%) |
Note: Surgery, NOS∗: a surgery procedure to the primary site was done, but no information on the type of surgical procedure is provided. Tumor destruction∗∗: tumor destruction procedure other than ablation.
As for the treatment trend across the 2004–2009 and 2010–2013 groups, the tumor resection rate decreased from 24.11% to 21.60%, while the ablation rate increased slightly from 1.39% to 1.56% (Table 1). The rate of patients undergoing radiotherapy, lymphadenectomy and distant site surgery was almost unchanged (Table 1). Among all 4642 patients, 3661 all-cause deaths and 2805 ICC-specific deaths were observed. The 12-, 60-, and 96-months estimated overall ICC-specific survival rates were 40.38%, 12.84%, and 10.38%, respectively (Supplementary Figure 1 and Supplementary Table 1).
Risk Factors for Survival
To evaluate the risk factors for survival, including TNM stages (AJCC 7th edition), for 2248 patients diagnosed between 2010 and 2013 were analyzed. As shown in Table 2, both univariate and multivariate Cox regression analyses showed age, being male and of the black race, poorly differentiated tumors and higher AJCC stages as significant risk factors for both all-cause death and ICC-specific mortality. Racial disparity existed and black patients had a higher risk of death than white patients. For surgery, both tumor resection and tumor destruction, including ablation, could significantly improve survival (Supplementary Figure 2 and Supplementary Table 2). Except for cancer-directed surgery, both radiotherapy and surgery at distant metastatic sites decreased the hazards of all-cause mortality and cancer-specific mortality in both univariate and multivariate Cox models (Table 2). However, lymphadenectomy did not show significant benefits to survival in the multivariate COX model.
Table 2.
Risk factors for survival in 2010–2013: outcome is all-cause mortality and cancer-specific mortality.
| All-cause mortality (n = 2248) |
Cancer-specific mortality (n = 1905) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox regression∗ | Multivariate Cox regression∗∗ | Univariate Cox regression∗ | Multivariate Cox regression∗∗ | ||||||
| Variables | Level | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | p-value | HR (95% CI) | P-value |
| Age | 1.024 (1.020, 1.028) | <.0001 | 1.024 (1.019, 1.029) | <.0001 | 1.021 (1.016, 1.026) | <0.0001 | 1.022 (1.016, 1.028) | <.0001 | |
| Sex | Male | 1.252 (1.130, 1.387) | <.0001 | 1.365 (1.212, 1.537) | <.0001 | 1.224 (1.090, 1.376) | 0.0007 | 1.311 (1.144, 1.502) | <.0001 |
| Female | Ref | Res | Ref | Ref | |||||
| Race | Black | Ref | Ref | Ref | Ref | ||||
| White | 0.836 (0.696, 1.003) | .05 | 0.768 (0.622, 0.947) | .01 | 0.815 (0.667, 0.995) | 0.04 | 0.713 (0.565, 0.899) | .004 | |
| Chinese | 0.757 (0.534, 1.073) | .12 | 0.645 (0.432, 0.962) | .03 | 0.716 (0.483, 1.063) | 0.09 | 0.623 (0.397, 0.978) | .04 | |
| Others | 0.824 (0.657, 1.033) | .09 | 0.716 (0.550, 0.931) | .01 | 0.849 (0.663, 1.088) | 0.20 | 0.689 (0.514, 0.924) | .01 | |
| Grade | Well differentiated | Ref | Ref | Ref | Ref | ||||
| Moderately differentiated | 1.038 (0.755, 1.429) | .82 | 1.265 (0.898, 1.782) | .22 | 1.058 (0.723, 1.549) | 0.77 | 1.373 (0.907, 2.077) | .13 | |
| Poorly differentiated | 2.058 (1.503, 2.818) | <.0001 | 1.986 (1.417, 2.785) | <.0001 | 2.218 (1.527, 3.221) | <0.0001 | 2.232 (1.488, 3.348) | <.0001 | |
| Undifferentiated | 2.098 (0.896, 4.914) | .09 | 2.136 (0.902, 5.060) | .08 | 2.188 (0.852, 5.616) | 0.10 | 2.295 (0.880, 5.987) | .09 | |
| T-stage | T0 | 2.087 (0.931, 4.678) | .07 | 1.175 (0.517, 2.671) | .70 | 2.140 (0.883, 5.187) | 0.09 | 1.079 (0.438, 2.657) | .87 |
| T1 | Ref | Ref | Ref | Ref | |||||
| T2 | 1.529 (1.343, 1.742) | <.0001 | 1.171 (0.954, 1.436) | .13 | 1.627 (1.399, 1.890) | <0.0001 | 1.174 (0.934, 1.476) | .17 | |
| T3 | 1.534 (1.291, 1.822) | <.0001 | 1.020 (0.789, 1.319) | .88 | 1.671 (1.375, 2.031) | <0.0001 | 1.010 (0.758, 1.345) | .95 | |
| T4 | 1.238 (1.006, 1.523) | .04 | 0.979 (0.738, 1.299) | .45 | 1.295 (1.021, 1.641) | 0.03 | 0.980 (0.716, 1.342) | .90 | |
| N-stage | N0 | Ref | Ref | Ref | Ref | ||||
| N1 | 1.306 (1.164, 1.465) | <.0001 | 1.044 (0.874, 1.247) | .63 | 1.379 (1.210, 1.571) | <0.0001 | 1.074 (0.882, 1.307) | .48 | |
| M-stage | M0 | Ref | Ref | Ref | Ref | ||||
| M1 | 2.021 (1.822, 2.242) | <.0001 | 1.198 (0.975, 1.472) | .09 | 2.237 (1.987, 2.518) | <0.0001 | 1.285 (1.020, 1.618) | .03 | |
| AJCC stage | I | Ref | Ref | Ref | Ref | ||||
| II | 1.585 (1.319, 1.905) | <.0001 | 1.323 (1.002, 1.747) | .05 | 1.687 (1.352, 2.107) | <0.0001 | 1.388 (1.005, 1.916) | .05 | |
| III | 1.846 (1.413, 2.411) | <.0001 | 1.665 (1.140, 2.431) | .008 | 2.072 (1.520, 2.823) | <0.0001 | 1.782 (1.157, 2.743) | .009 | |
| IV | 2.280 (1.967, 2.642) | <.0001 | 1.434 (1.072, 1.917) | .02 | 2.644 (2.214, 3.156) | <0.0001 | 1.398 (1.004, 1.947) | .05 | |
| Cancer-directed | None | Ref | Ref | Ref | Ref | ||||
| surgery | Resection | 0.215 (0.183, 0.254) | <.0001 | 0.263 (0.205, 0.339) | <.0001 | 0.192 (0.158, 0.232) | <0.0001 | 0.238 (0.176, 0.322) | <.0001 |
| Ablation | 0.264 (0.156, 0.448) | <.0001 | 0.289 (0.166, 0.504) | <.0001 | 0.238 (0.127, 0.443) | <0.0001 | 0.250 (0.128, 0.487) | <.0001 | |
| Tumor destruction | 0.285 (0.107, 0.762) | .01 | 0.261 (0.083, 0.813) | .02 | 0.313 (0.117, 0.836) | 0.02 | 0.300 (0.096, 0.939) | .04 | |
| Regional lymph | None | Ref | Ref | Ref | Ref | ||||
| node surgery | 1–3 LN removed | 0.263 (0.201, 0.345) | <.0001 | 0.870 (0.615, 1.231) | .43 | 0.249 (0.184, 0.339) | <0.0001 | 0.930 (0.622, 1.392) | .73 |
| ≥4 LN removed | 0.384 (0.293, 0.505) | <.0001 | 1.319 (0.908, 1.915) | .15 | 0.368 (0.270, 0.501) | <0.0001 | 1.404 (0.916, 2.152) | .12 | |
| LN removed, unknown N | 0.344 (0.129, 0.919) | .03 | 0.370 (0.117, 1.169) | .09 | 0.265 (0.066, 1.062) | 0.06 | 0.377 (0.092, 1.534) | .17 | |
| Surgery at | No | Ref | Ref | Ref | Ref | ||||
| distant sites | Yes | 0.467 (0.294, 0.744) | .001 | 0.347 (0.189, 0.639) | .0007 | 0.496 (0.303, 0.812) | 0.005 | 0.364 (0.193, 0.688) | .002 |
| Radiation | No | Ref | Ref | Ref | Ref | ||||
| Yes | 0.600 (0.511, 0.706) | <.0001 | 0.587 (0.485, 0.709) | <.0001 | 0.606 (0.504, 0.728) | <0.0001 | 0.598 (0.482, 0.742) | <.0001 | |
Note: ∗Univariate Cox regression analyses were performed to evaluate the risk factors for all-cause mortality and breast cancer-specific mortality. ∗∗Multivariate Cox regression analyses were performed by adjusting all the candidate variables listed.
Benefits of RFA and Radiation on Survival
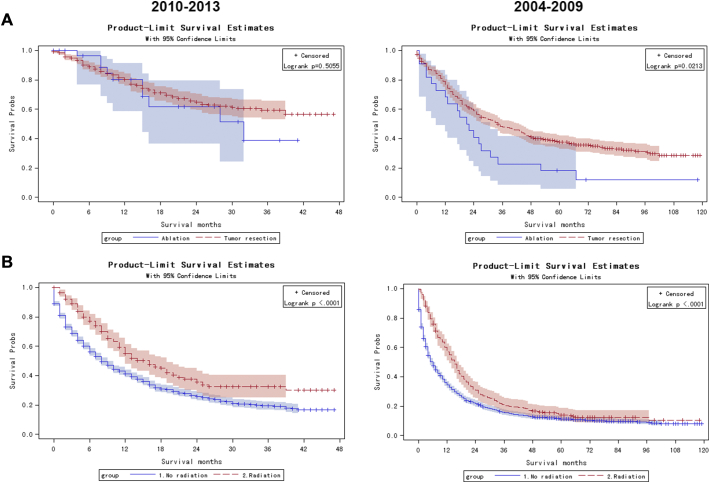
As shown in Figure 2A, compared to patients with tumor resection, patients who underwent ablation had a poorer prognosis in 2004–2009 (P = .0213), but showed a similar Kaplan–Meier curves as the tumor resection group in patients diagnosed in 2010–2013 (P = .5055). Radiotherapy could significantly promote cancer-specific survival in both patients diagnosed in 2010–2013 (log-rank P < .0001) and in 2004–2009 (log-rank P < .0001, Figure 2B). These results suggested that radiotherapy performed better than patients without radiation and lymphadenectomy. In 2010–2013, ablation showed similar effect on overall cancer-specific survival to tumor resection.
Figure 2.
Kaplan–Meier curves among patients diagnosed from 2010 to 2013 and 2004 to 2009.
Kaplan–Meier curves among patients treated by resection (red dash line) and ablation (blue line) for cancer-specific mortality
Kaplan–Meier curves among patients treated with radiotherapy (red dash line) and without radiotherapy (blue line) for cancer-specific mortality.
Stratified Cox Model
Because the number of patients with tumor destruction (including ablation) was small, no valid hazards ratios (HR) could be generated by the stratified Cox regression model. To further evaluate the roles of radiotherapy on survival in resection patients and unresectable patients who did not receive tumor-directed surgery, stratified Cox regression models were used. As demonstrated in Table 3, compared to the non-radiotherapy group, radiation was associated with a better ICC-specific survival in patients who did not receive any cancer-directed surgery (P < .0001). But for patients with tumor resection or RFA (ablation), radiation did not show significant survival benefit (P = .73 and 0.46, respectively). For other stratified subgroups, radiation showed significant survival benefits in all age, sex and TNM stage subgroups.
Table 3.
Role of radiation related to cancer-specific death in stratified Cox regression analysis
| Patient category∗ | Level | Cancer-specific death⁎⁎ |
HR (95% CI)⁎⁎⁎ | P-value | Interaction term |
|
|---|---|---|---|---|---|---|
| Radiation | No radiation | P-value # | ||||
| Cancer-directed surgery | No cancer-directed surgery | 312/424 | 2194/2614 | 0.61 [0.54, 0.69] | <.0001 | .002 |
| Tumor resection | 82/166 | 361/816 | 1.00 [0.79, 1.27] | .73 | ||
| Ablation | 3/5 | 28/53 | 0.66 [0.20, 2.19] | .46 | ||
| Age | <70 y | 282/429 | 1555/2189 | 0.72 [0.63,0.81] | <.0001 | .37 |
| ≥70 y | 118/170 | 1060/1337 | 0.66 [0.54, 0.79] | <.0001 | ||
| Sex | Female | 204/316 | 1282/1770 | 0.66 [0.57, 0.77] | <.0001 | .55 |
| Male | 196/283 | 1333/1756 | 0.71 [0.61,0.82] | <.0001 | ||
| T stage | T0 | 0/2 | 11/11 | - | - | .99 |
| T1 | 111/196 | 747/1139 | 0.71 [0.58, 0.87] | .0008 | ||
| T2 | 93/157 | 580/845 | 0.71 [0.57, 0.88] | .002 | ||
| T3 | 100/122 | 551/668 | 0.72 [0.58, 0.89] | .003 | ||
| T4 | 59/76 | 289/370 | 0.65 [0.49, 0.87] | .003 | ||
| N stage | N0 | 240/389 | 1645/2332 | 0.68 [0.60, 0.78] | <.0001 | .91 |
| N1 | 128/175 | 666/845 | 0.65 [0.53, 0.78] | <.0001 | ||
| M stage | M0 | 256/424 | 1367/2057 | 0.73 [0.64, 0.84] | <.0001 | .73 |
| M1 | 144/175 | 1248/1469 | 0.76 [0.64, 0.90] | .002 | ||
Note:
Patient category∗: patients were categorized by diagnosis time (2004–2009, 2010–2013) and N0 / N1 stages. N0 category included patients with no lymph node metastasis, while N1 category included patients with lymph node metastasis.
Cancer-specific death⁎⁎ was demonstrated as ICC-specific death number (numerator) divided by number of patients treated by radiation/no radiation in each patients category∗ with or without primary tumor surgery.
HR (95% CI)∗∗∗ showed the hazard ratio (95% confidence interval) of cancer-specific death among radiation patients vs no radiation patients in subgroups.
P-value # for interaction term indicated the different effect of radiation among stratified subgroups.
Discussion
The incidence rate of ICC in the United States has increased over the last four decades (1973–2012), from 0.44 to 1.18 cases per 100,000, and this trend has accelerated during the past decade [14]. The mortality from ICC also increased markedly. The age-adjusted mortality rate per 100,000 persons for whites and blacks increased from 0.14 and 0.15 (1975–1979) to 0.65 and 0.58 (1993–1997), respectively [15]. This tumor is associated with a poor prognosis [16]. Based on the 2004–2013 SEER data included in this study, the overall 60-month (5-year) all-cause survival and cancer-specific survival was 9.7% and 12.8%, respectively (Supplementary Figure 1). The 60-month (5-year) survival of the ablation group, tumor resection group and non-surgery group was 23.25%, 40.31% and 2.97%, respectively (Supplementary Table 2).
The AJCC 7th edition TNM staging system has, for the first time, attributed a unique pTNM staging to ICC and provided routine lymphadenectomy at the time of surgery for ICC, which becomes the standard of care [3], [5]. According to AJCC TMN Staging (7th ed., 2010), all tumors with periductal invasion (T4), lymph node metastases (N1) or distant metastasis (M1) are categorized as stage IV. In this study, we found that compared to the AJCC 6th edition, more patients were categorized into stage IV under the AJCC 7th staging system (Tables 1, 58.67% vs 37.39%). Additionally, there are more non-surgery patients diagnosed from 2010 to 2013 (76.1%), compared to those from 2004 to 2009 (72.9%) (P = .01). Here, since more than 70% of ICC tumors were inoperative and the 60-month (5-year) survival for this portion of patients was less than 3%, we believe that we should focus on the treatment of unresectable ICC patients.
In NCCN guidelines, current primary options for metastatic disease only include clinical trials, fluoropyrimidine- or gemcitabine-based therapy [17] and supportive care. Currently, clinical trial and fluoropyrimidine / gemcitabine-based chemotherapy are the primary treatment options for this group of patients [18]. For patients with unresectable tumors, locoregional tumor destruction is suggested by NCCN guidelines.
Locoregional therapy, including RFA, is effective in unresectable ICC with a tumor size less than 3–5 cm [8], [19]. In this study, we suggested that RFA would help improve survival for unresectable patients. Here, we found that the proportion of ablation in the tumor destruction group increased tremendously from 25% in 2004 to 73.33% in 2013 (Supplementary Figure 3). Moreover, even Kaplan–Meier analysis suggested a better survival in resection patients than ablation patients in 2004–2009 (P = .02); this gap disappeared in patients diagnosed from 2010 to 2013 (P = .51, Figure 2A).
According to NCCN guidelines, radiation combined with chemotherapy is recommended for patients with microscopic tumor margins (R1) or positive regional lymph node(s) after cancer-directed resection [20], [21]. However, in this study, radiation did not show benefits to cancer-specific survival in the tumor resection group in the stratified Cox model (Table 3). Instead, in the non-surgery group, radiotherapy provided a significant protective effect against cancer-specific death (P < .0001). These results were consistent with previous study, which show that radiotherapy did not provide a survival benefit for resection patients with positive resection margins and node negative ICC [22]. Recent studies also demonstrated the benefits of radiation in unresectable ICC [10], [23], [24].
Lymph node metastasis is an important prognostic indicator of survival, and lymphadenectomy is routinely considered at operation. Lymphadenectomy has a survival benefit and is thus suggested to be considered for all patients [12]. A portal lymphadenectomy is reasonable as it provides relevant staging information. However, based on SEER data between 1988 and 2011, lymphadenectomies only showed a therapeutic benefit among a subset of patients (males, age ≤ 60 years and tumor size >50 mm) [25], [26]. Even some reports suggested that lymphadenectomy has no therapeutic benefit to patients undergoing surgery [26], [27], [28], [29]. Extended lymphadenectomy patients had even worse overall survival compared with patients with more limited lymph node resection [30]. In this study, patients with lymphadenectomy had a significantly better survival than patients without lymphadenectomy (Figure 2C). Stratified Cox regression analysis showed that lymphadenectomy was proven to have no benefits to cancer-specific survival for patients with surgery, bu5 showed a significant benefit to survival in patients without primary tumor surgery (Table 3). For both N0 and N1 non-surgery patients, the odds of cancer-specific mortality among patients having lymphadenectomy were significantly lower than the odds of cancer-specific mortality among patients having no lymphadenectomy (P = .01 for N0 patients, P = .003 for N1 patients). These results suggested that for unresectable patients, portal lymphadenectomy might not only provide staging information, but also help relieve portal tumor burden and prolong survival.
With the advanced CT/PET techniques, the sensitivity in detecting regional lymph nodes metastases and distant metastases increased significantly [31]. The rate of lymph node(s) metastases and distant metastases increased from 23.54% and 37.4%, respectively, from 2004 to 2009 to 30.58% and 40.5%, respectively, from 2010 to 2013 (Table 1). Even distant metastasis is a key contra-indicator for primary tumor resection; still, 2–4% stage IV patients received surgery of distant lymph node(s) or metastatic sites (Table 2). For unresectable ICC patients, current treatment strategies are limited. Aggressive treatment might help relieve tumor burden and bring survival benefits to patients.
In this study, we investigated several currently-available methods, including RFA, radiotherapy, and lymphadenectomy. Future clinical research should focus more on this group of patients and develop more effective methods to improve survival of ICC patients.
The SEER program of the National Cancer Institute (NCI) is a population-based cancer registry covering approximately 30% of the population in the United States. This database is the largest publicly available and authoritative information source on cancer incidence and survival. Using this reliable and large-scale research dataset, we could get useful information to guide clinical anti-cancer treatment for ICC.
Conclusions
In conclusion, compared to patients staged according to AJCC 6th edition staging system from 2004 to 2009, more stage IV patients were diagnosed from 2010 to2013 according to the AJCC 7th edition staging system. The percentage of people with tumor resection decreased between 2010 and 2013 compared to patients diagnosed from 2004 to 2009. Among patients who did not receive tumor resection, RFA, radiotherapy, and lymphadenectomy showed benefits to cancer-specific survival, especially in patients diagnosed from 2010 to 2013.
The following are the supplementary data related to this article.
Supplementary material 1
Supplementary material 2
Trend for treatment.
Declarations
Ethics approval and consent to participate
SEER database was approved by the National Cancer Institute (NIH) Ethics Committee. NIH authorized SEER database to serve as a research resource to general research community.
Consent to Publish
SEER database is an open-source database. The consent to publish form is not available.
Availability of Data and Materials
The SEER*Stat database, which was released by the Surveillance Research Program at the NCI in 2017, was used as the data source in the present study [32]. This study used SEER*Stat database released in the SEER 18Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying) incidence database. Data source was available for general populations.
Conflict of Interest Disclosures
None reported
Funding/Support
We acknowledged funding from The New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (JY201519) to Hongbo Xu.
Authors' Contributions
JL designed this study and interpreted the outcomes regarding surgery and survival. JS helped perform the data analysis and prepare the manuscript. ZH wrote the manuscript, performed the data analysis. All authors had read and approved the final manuscript.
Acknowledgements
This study used the SEER 18 Regs research database as the data source. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the SEER Program tumor registry; and the Information Management Service Inc. for the creation and distribution of the SEER*Stat database.
Contributor Information
Jieqiong Liu, Email: liujieqiong8708@163.com.
Meizuo Zhong, Email: zhongmeizuo@188.com.
Yuhua Feng, Email: fyh87256630@csu.edu.cn.
Shan Zeng, Email: shanzeng2012@qq.com.
Yikai Wang, Email: yikai.wang@emory.edu.
Hongbo Xu, Email: xu.hongbo@csu.edu.cn.
Hui Zhou, Email: zhouhui9403@126.com.
Reference
- 1.Nakagohri T, Asano T, Kinoshita H, Kenmochi T, Urashima T, Miura F, Ochiai T. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–293. doi: 10.1007/s00268-002-6696-7. [DOI] [PubMed] [Google Scholar]
- 2.Konstadoulakis MM, Roayaie S, Gomatos IP, Labow D, Fiel MI, Miller CM, Schwartz ME. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, Nuzzo G, Belghiti J, Pruvot FR, Regimbeau JM. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 6.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2010;26:269–273. doi: 10.1097/MOG.0b013e328337c899. [DOI] [PubMed] [Google Scholar]
- 7.Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, Fugazzola C. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205–W209. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 9.Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol. 2014;110:163–170. doi: 10.1002/jso.23605. [DOI] [PubMed] [Google Scholar]
- 10.Jackson MW, Amini A, Jones BL, Rusthoven CG, Schefter TE, Goodman KA. Treatment selection and survival outcomes with and without radiation for unresectable. Localized Intrahepatic Cholangiocarcinoma Cancer J. 2016;22:237–242. doi: 10.1097/PPO.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 11.Jia AY, Wu JX, Zhao YT, Li XY, Wang Z, Rong WQ, Wang LM, Jin J, Wang SL, Song YW. Intensity-modulated radiotherapy following null-margin resection is associated with improved survival in the treatment of intrahepatic cholangiocarcinoma. J Gastrointest Oncol. 2015;6:126–133. doi: 10.3978/j.issn.2078-6891.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribero D, Pinna AD, Guglielmi A, Ponti A, Nuzzo G, Giulini SM, Aldrighetti L, Calise F, Gerunda GE, Tomatis M. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 13.Wingo PA, Jamison PM, Hiatt RA, Weir HK, Gargiullo PM, Hutton M, Lee NC, Hall HI. Building the infrastructure for nationwide cancer surveillance and control—a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States) Cancer Causes Control. 2003;14:175–193. doi: 10.1023/a:1023002322935. [DOI] [PubMed] [Google Scholar]
- 14.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: Intrahepatic disease on the rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 16.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Borbath I, Ceratti A, Verslype C, Demols A, Delaunoit T, Laurent S, Deleporte A, Vergauwe P, Van Maanen A, Sempoux C. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. 2013;24:2824–2829. doi: 10.1093/annonc/mdt337. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, Heo DS, Bang YJ. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008;8:374. doi: 10.1186/1471-2407-8-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:324–328. doi: 10.1097/MOG.0b013e32835d9dea. [DOI] [PubMed] [Google Scholar]
- 20.Gil E, Joh JW, Park HC, Yu JI, Jung SH, Kim JM. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol. 2015;13:227. doi: 10.1186/s12957-015-0637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S, Kim K, Chie EK, Kim S, Park HJ, Yi NJ, Suh KS, Ha SW. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Clin Transl Oncol. 2015;17:825–829. doi: 10.1007/s12094-015-1312-0. [DOI] [PubMed] [Google Scholar]
- 22.Hammad AY, Berger NG, Eastwood D, Tsai S, Turaga KK, Christian KK, Johnston FM, Pawlik TM, Gamblin TC. The Impact of Surgical Margins and Lymph Node Status on Survival. 2016. Is radiotherapy warranted following intrahepatic cholangiocarcinoma resection? (Ann Surg Oncol). [DOI] [PubMed] [Google Scholar]
- 23.Jia Z, Paz-Fumagalli R, Frey G, Sella DM, McKinney JM, Wang W. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: preliminary results. J Cancer Res Clin Oncol. 2017;143(3):481–489. doi: 10.1007/s00432-016-2291-4. [DOI] [PubMed] [Google Scholar]
- 24.Cuneo KC, Lawrence TS. Growing evidence supports the use of radiation therapy in unresectable intrahepatic cholangiocarcinoma. Cancer J. 2016;22:243–244. doi: 10.1097/PPO.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 25.Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2016;113:685–691. doi: 10.1002/jso.24213. [DOI] [PubMed] [Google Scholar]
- 26.Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015;50:913–927. doi: 10.1007/s00535-015-1071-2. [DOI] [PubMed] [Google Scholar]
- 27.Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 28.Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, Kendrick ML, Huebner M, Que FG. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford). 2011;13:612–620. [DOI] [PMC free article] [PubMed]
- 29.Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015;157:666–675. doi: 10.1016/j.surg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Hakeem AR, Marangoni G, Chapman SJ, Young RS, Nair A, Hidalgo EL, Toogood GJ, Wyatt JI, Lodge PA, Prasad KR. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil-lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Eur J Gastroenterol Hepatol. 2014;26:1047–1054. doi: 10.1097/MEG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, Lee SS, Seo DW, Lee SK. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 32.Software. Surveillance Research Program, National Cancer Institute SEER*Stat software. www.seer.cancer.gov/seerstat. 2016;version 8.3.2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Trend for treatment.
Data Availability Statement
The SEER*Stat database, which was released by the Surveillance Research Program at the NCI in 2017, was used as the data source in the present study [32]. This study used SEER*Stat database released in the SEER 18Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying) incidence database. Data source was available for general populations.